Fabry disease (OMIM 301500) is an X-linked genetic disorder where lysosomal α-galactosidase A(α-Gal A) is deficient, leading to the accumulation of globotriaosylceramide and/or globotriaosyl-sphingosine (lyso-Gb3) especially in the kidneys, heart, eyes and biological fluids.1,2 The major clinical manifestations are chronic pain, gastrointestinal disturbance, angiokeratom, anhidrosis, and acroparesthesias in earlier stage and cardiomyopathy, cerebrovascular disease, and renal insufficiency in the later stage.1 Clinical symptoms and the severity of the disease roughly correlate with residual α-Gal A activity.1 Diagnosis of affected hemizygote can be readily achieved by molecular analysis or α-Gal A assay however, a significant number of affected Fabry heterozygotes show below the normal range of α- Gal A activities where enzymatic diagnosis is unrealiable.2 Enzyme replacement therapy (ERT) on early stage could be optimally effective, therefore timely screening and diagnosis must be initiated because it is crucial for the quality of life.
Up until a few years ago, generally Gb3 level in plasma and/or urine has been received major attention as a biomarker for Fabry disease.3-5 Recently, researcher have paid attention to the excretion of plasma lyso-Gb3 level and published it’s significant correlation with clinical severity of disease manifestations.6,7,9-11
Traditionally analytical method of lyso-Gb3 was based on O-phthalaldehyde derivatization of amine in the lysoglycolipid followed by HPLC-fluorescence detection.11-14 LC-MS/MS has it’s own strengths such as high sensitivity, specificity and rapidity of analysis, thus the paradigm of the analysis is getting shifted to the usage of LC-MS/MS including lyso-Gb3.9,15-19 This study is devised to develop and validate a fast and simple analytical method for quantification of lyso-Gb3 in human plasma using UPLCESI- MS/MS.
Lyso-Gb3 and N,N-Dimethyl-D-erythro-sphingosine (internal standard) was obtained from Matreya (PA, USA). All other chemicals and organic solvents, dimethylsulfoxide (DMSO) and acetonitrile (ACN) were of analytical-reagent grade and purchased from J. T. Baker or YAKURI pure chemical Co. LTD (Osaka, Japan). Distilled water was prepared by Millipore-Milli QTM. TAITEC model DTU-2C Thermo vap (Tokyo, Japan) was used for evaporation.
>
Preparation of standard solution
Each of stock solution (1 mg/mL of lyso-Gb3) and internal standard solution (5 mg/mL) were prepared by dissolving in dimethylsulfoxide, stored at -20℃ until the analysis, and were stable for at least 6 months. Working solutions (100 ng/mL) were prepared by serial dilution of stock solutions in 50% ACN as needed.
Electrospray ionization (ESI)-MS/MS analyses were performed on Applied Biosystem API 4000 MS/MS (CA, USA) coupled with Shiseido corporation UPLC system (Tokyo, Japan). It consisting of a model LC-3133 Nonspace autosampler and 3101 binary pump. Multiple reaction monitoring (MRM) in positive ion mode was used for quantification in MS/MS (declustering potential of 126 V, collision energy of 49 V, collision exit potential of 16 V, and the entrance potential of 10 V).
Chromatographic separation was carried out on Phenomenex Kinetex-C18 (2.1 × 50 mm, 2.6 μm). The mobile phase consisted of 0.1% formic acid (FA) in 5% ACN (A) and 0.1% FA in ACN (B) ) as a flow rate of 300 μL/min. Fast gradient elution was achieved with starting at 20% solvent B up to 90% solvent B. The autosampler was maintained at 4℃ and the injection volume was 10 μL with loop.
>
Specimen collection and preparation
Plasmas of human control and Fabry disease patient were obtained from Seoul Medical Science Institute (Seoul, Korea) in 30 healthy volunteers (male, n=15; female n=15) and Seoul Asan Medical Center (Seoul, Korea) with informed consent, respectively. All the samples were immediately stored at -20℃ until the analyses.
Human plasma (100 μL) was simply treated by protein precipitation with ACN. To supernatant 200 μL of working solution and 10 μL of IS (100 ng/mL) was added. A total of 690 μL of ACN as an extraction solvent was added to the mixture and vortexed for 1 min. After centrifugation at 12,000 g for 5 min, supernatant passed through a 0.2-μm filter (Whatman, NJ, USA) before injection (10 μL).
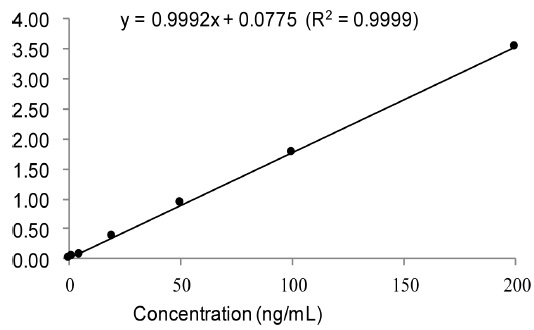
Calibration curves were plotted by the peak area ratio of analyte to IS against the analyte concentration using control human pooled plasmas (100 μL) spiked at various concentrations. The concentration of spiked to plasmas were 2, 5, 20, 50, 100, and 200 ng/mL for working solution.
The limit of detection (LOD) was calculated where the S/N ratio was greater than 3 and the limit of quantitation (LOQ) was greater than 10.20 The LOQ was validated with the lowest concentration where the lyso-Gb3 could be quantified with acceptable accuracy (< 20%) and precision (< 20% CV). The LOQ was confirmed by the inclusion of the lowest concentration in the range of the calibration curves.20
>
Validation; Accuracy, Precision and Recovery
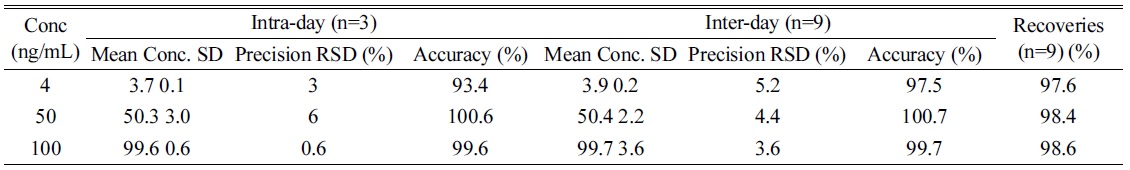
Method validation was carried out the United States Food and Drug Administration’s bioanalytical method validation references.20 Accuracy was determined as the ratio of back-calculated value to the nominal standard concentration and precision as the % CV of peak areas from replicate analyses for intra-day (n = 3) and for interday (n = 9). Accuracy was calculated as: [measured concentration] / [nominal concentration] × 100%.21
The recovery was determined by calculating the concentration of extracted and non-extracted spiked samples at 3 levels of concentration. Carryover effect was evaluated such that six blank samples were run without any residual peak after running high concentration of lyso-Gb3 (data not shown).
The ACN have been chosen for the most efficient among the solvents tested (hexane, diethylether, and dicholomethane) for protein precipitation. Using only simple protein precipitation was less expensive and more rapid than LLE and SPE. After deproteinization, the supernatant was filtered and injected to the UPLC system.
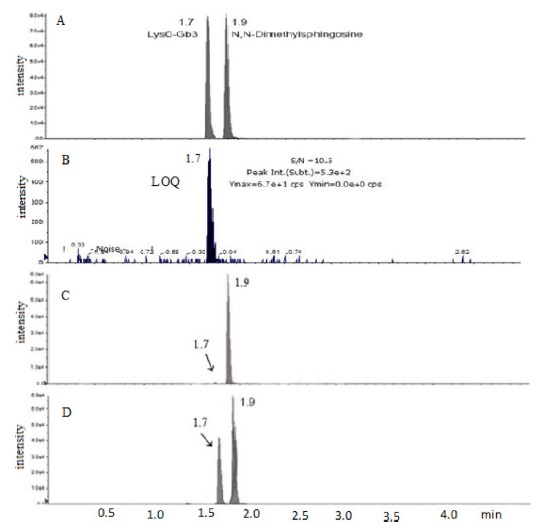
Base peak [M+H] in mass spectrum was obtained at m/z = 786.6 and product ions were m/z = 282.4, m/z = 264.4, m/z = 96 and m/z = 84 as in order of fragmentation. Chromatograms of lyso-Gb3 and internal standard were obtained within 2 min (Figure 1 A). The calibration curve of spiked pooled plasma are shown where serial dilution of lyso-Gb3 standard is added to. The calibration curve showed an excellent linearity over the range from 2 to 200 ng/mL with R2 = 0.9999 (y = 0.9992x + 0.0775) (Figure 2). A new method required within 15 min from the sample preparation to the chromatographic analysis for entire analysis.
The LOD of 1.27 nmol/L (~1 ng/mL, S/N = 3.5) determined in our method is well below the LOD reported in Togawa
[Table 1.] Precision, accuracy and recovery of the intra-day and inter-day assay.
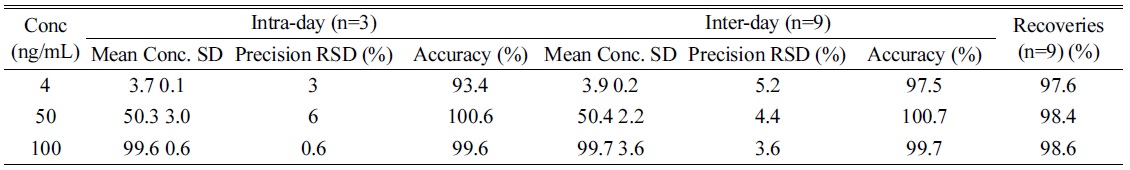
Precision, accuracy and recovery of the intra-day and inter-day assay.
The developed UPLC-MS/MS method was then applied to the real human plasma with and without Fabry disesase. Figure 1 shows representative UPLC-MS/MS chromatograms of lyso-Gb3 standard and internal standard (Figure 1 A), lyso-Gb3 at the LOQ concentration (Figure 1 B), control plasma (Figure 1 C), and plasma with Fabry disease patient (Figure 1 D). High excretion of lyso-Gb3 was observed in plasma with Fabry disease compared to that of control plasmas as expected (Figure 1 C and D).
When measured control human plasma (n = 30), normal range of lyso-Gb3 in Korean was observed as 0.19 - 1.97 ng/mL whereas Fabry disease plasma (n = 3) was determined as 12.90 - 49.40 ng/mL. Togawa
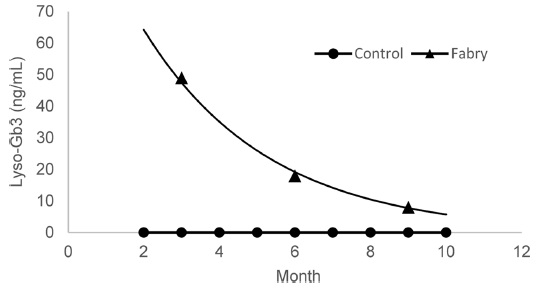
To evaluate the clinical correlation with lyso-Gb3 level as well as therapeutic drug monitoring after ERT, the levels of the plasma with one Fabry disease patient were kept tracking at every 3 month during the 9 months of ERT (Figure 3). The results show a close correlation between clinical symptom and lyso-Gb3 level.
Recent report suggested that the lyso-Gb3 level has more correlation with clinical symptom in patients with Fabry disease.23 The deacylated Gb3 analogue lyso-Gb3 was found remarkably in high amount in plasma and urine of Fabry disesase patient, lyso-Gb3 could be a potential biomarker for the diagnosis and monitoring of Fabry disease.9,10,18 So far ERT is the only treatment available for Fabry disease however a new approach for the treatment of Fabry disease was investigated using pharmacological chaperone therapy and found to be effective.24-27
Nontheless, screening for Fabry disease as early as possible is strongly suggested for the quality of life. This study proved the possibility of screening for Fabry disease with a simple and rapid analytical method using UPLC-MS/MS.
A rapid, simple, and sensitive analytical method was developed for the determination of lyso-Gb3 by UPLCMS/ MS without expensive and tedious solid phase extraction step. The method was successfully validated and applied to the human plasma. Thus, this method could be a useful screening and a therapeutic monitoring tool for Fabry disease.