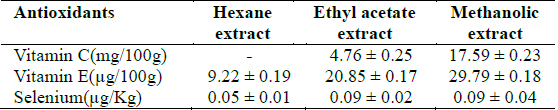Malaria is still the most prevalent and deadly parasitic disease in the world, infecting between 300 and 500 million people every year and causing about a million deaths annually, mostly among young children in Sub-Saharan Africa (McCombie, 2002). Malaria induces oxidative stress through an increased production of ROS within and outside the parasitized erythrocyte. Oxidative stress is the result of a disturbance in the balance of naturally generated oxidants and antioxidants. This can be caused by an increase in the production of reactive oxygen species (ROS) and/or decrease in the activity of antioxidant systems (Postma et al., 1996). Biologically, free radicals are known to exert some physiological functions in the body such as biosynthesis, detoxification and microorganism clearance (Braganza et al., 1995). Therefore, the antioxidant defense is supposed to maintain a normal homeostatic balance between free radical production and clearance. The defense against free radical in the body comprise a complex antioxidant system including vitamins A, E, C, glutathione and enzymatic antioxidants such as glutathione reductase (GR), glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) (Halliwell and Gutteridge, 1990).
The role of antioxidants in malaria patients is currently receiving attention; antioxidants have been found to influence host cellular and immunological functions (Spallhoiz et al., 1990, Terahima et al., 2002). There is also increasing evidence that natural antioxidants, especially those contained in some spices, herbs and medicinal plants, may be useful in preventing the deleterious consequences of oxidative stress (Noda et al., 1997). Coincidentally, due to the rapid emergence of widespread drug resistance to most currently available antimalarial drugs (Tona et al., 2001) and the high cost of the same, most people in rural and urban areas in Africa still rely on medicinal plants; about 80% of the population of Countries in Africa use traditional medicine to help meet some of their primary health care needs (WHO, 2001). One of such plants used in traditional herbal treatment of malaria is Clerodendrum violaceum.
Chloroquine diphosphate salt was obtained from Sigma Chemical Company, St. Louis, Mo, USA. n-Hexane, ethyl acetate and methanol were obtained from Eagle Scientific Limited, Nottingham. Assay kits for Superoxide dismutase, Glutathione peroxidase and Glutathione reductase were obtained from Randox Laboratories Ltd. (Co. Antrim, U.K). All other reagents used were of analytical grade and prepared in all glass distilled water.
One hundred and forty four adult Swiss albino mice with an average weight of 20 ± 2 g were obtained from the animal breeding unit of the Department of Biochemistry, University of Jos, Plateau State, Nigeria. The mice were housed in plastic cages and maintained under standard laboratory conditions with free access to rat pellets and tap water ad libitum. The research adhered to the Principles of Laboratory Animal Care (NIH publication #85−23, revised in 1985).
Fresh leaves of
Ethical clearance for the study was obtained from the Departmental postgraduate committee.
>
Determination of antioxidant contents
The total phenolic contents of the extracts were determined by using Folin-Ciocalteu reagent (Nabavi et al., 2008). Total flavonoid contents were determined according to colorimetric method of Chang et al. (2002). Vitamins C and E were assayed by the method described by AOAC (2005).
The free radical scavenging capacity of the extracts were analysed using the DPPH scavenging assay according to the methods of Shimada et al. (1992). The reducing power of the extracts was quantified by the method described by Yen et al. (1995). Hydrogen peroxide scavenging activities of the extracts were evaluated according to the method of Zhao et al. (2006). Moreover, their superoxide anion scavenging activities were assayed as per Yaping et al. (2003).
Extract administration
The effects of
>
Sample collection and preparation
Four animals in each group were sacrificed 24 h after the last extract/drug administration, using slight ether anaesthesia and were dissected. Blood was collected by cardiac puncture into clean, dry sample tubes containing EDTA and centrifuged at 2000 rpm for 5 min to remove plasma. The red blood cells were then washed by re-suspending in 0.9% NaCl and centrifuging for 5 min at 2000 rpm after each wash; they were lysed by resuspending in cold distilled water and stored frozen until required. The liver of each animal was also quickly removed, cleansed of superficial connective tissue and blood. They were then homogenized in ice-cold 0.25 M sucrose solution (1:5 w/v). The homogenates were stored frozen overnight to ensure maximum release of enzymes (Ngaha et al., 1989). On day 8 post-inoculation, the remaining animals were also sacrificed and the same samples were collected.
SOD activities in the erythrocytes and liver were assayed by the method of Marklund and Marklund (1974); those of CAT were assayed by the method of Aebi (1998). GPx activities in whole blood and liver were assayed by the method of Paglia and Valentine (1967) while GR activities in the erythrocytes and liver were carried out by the method described by Goldberg and Spooner (1983). Protein concentrations in the samples were determined using the Biuret method (Gornall et al., 1949).
The group means ± Standard Deviation (SD) for each parameter was calculated and significant differences were determined by Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test at 95% confidence level using SPSS-PC programme packages. (Version 16.0, SPSS Inc, Chicago)
>
Antioxidant constituents of extracts
The results revealed that the methanolic extract of
[Table 1.] Selenium and vitamins C and E concentrations in Clerodendrum violaceum leaf extracts
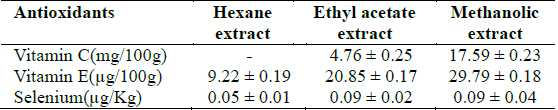
Selenium and vitamins C and E concentrations in Clerodendrum violaceum leaf extracts
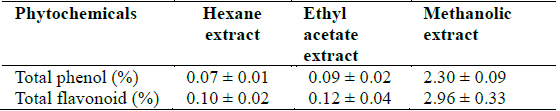
[Table 2.] Total phenol and flavonoid concentrations in Clerodendrum violaceum leaf extracts
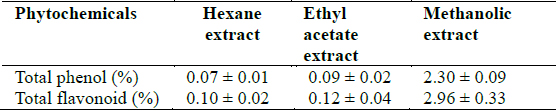
Total phenol and flavonoid concentrations in Clerodendrum violaceum leaf extracts
>
In vitro antioxidant activities
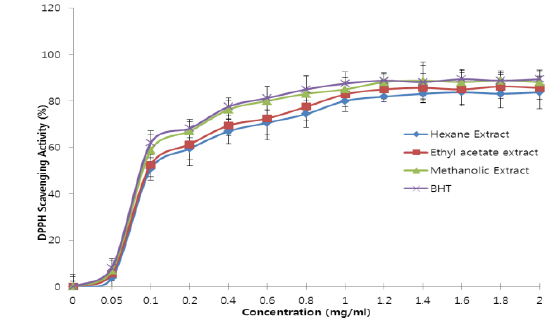
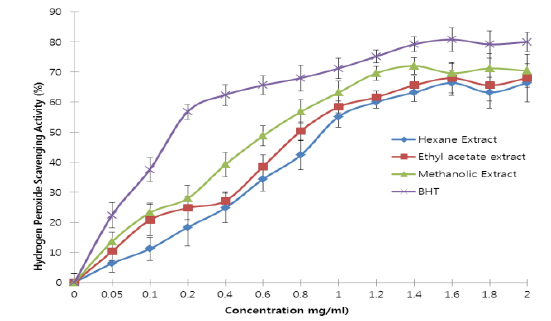
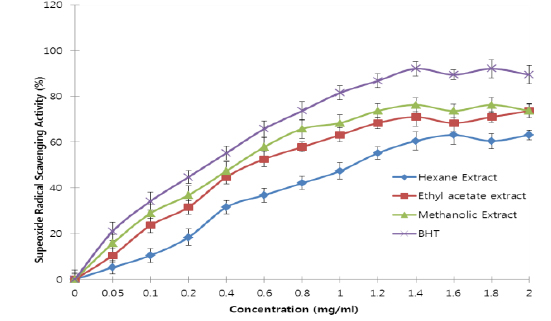
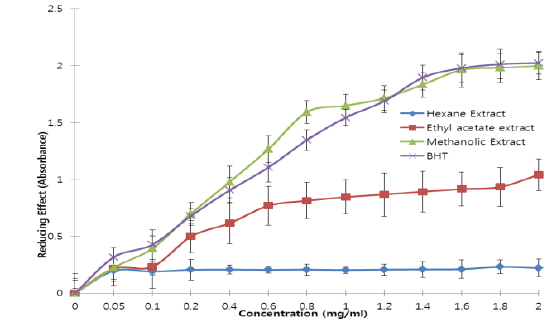
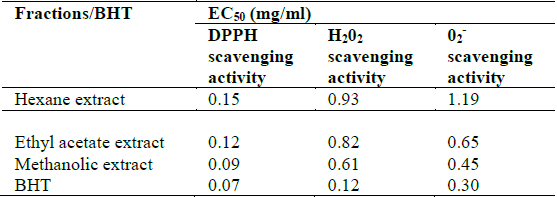
All the extracts exhibited antioxidant activities in vitro but their capabilities differed for the different indicators considered. For the DPPH radical scavenging activity, there was an increase in scavenging activity with increase in concentration for all extracts; however, the methanolic extract had the highest scavenging activity (88.75%), which compared favourably with that of Butylated HydroxyToluene (BHT) (89.38%), the standard (Fig. 1). Similar results were obtained for the hydrogen peroxide scavenging assay. There was an increase in scavenging activity with increase in concentration for all the extracts. The maximum scavenging activities were 66.4%, 68.0%, 72.0% and 80.8% for hexane extract, ethyl acetate extract, methanolic extract and BHT respectively (Fig. 2). Results for the superoxide radical scavenging activity also showed an increase in activity with increase in concentration for all the extracts, with the methanolic extract comparing favourably well with BHT (Fig. 3). The extracts, except the hexane extract, exhibited increased reducing power with increase in concentration. The methanolic extract had the highest increase, comparing favourably well with the standard (BHT). The hexane extract maintained a plateau at all concentrations (Fig. 4). On the overall, the methanolic extract had the lowest EC50 for the free radical scavenging activities (Table 3).
[Table 3.] In vitro antioxidant activities of Clerodendrum violaceum leaf extracts
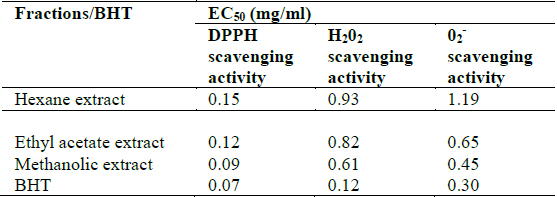
In vitro antioxidant activities of Clerodendrum violaceum leaf extracts
>
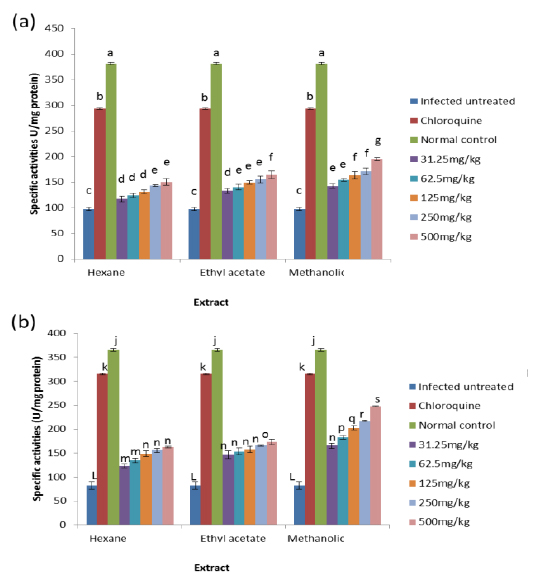
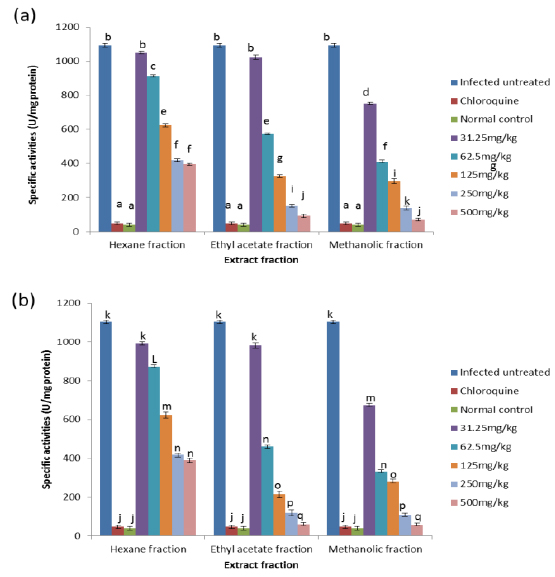
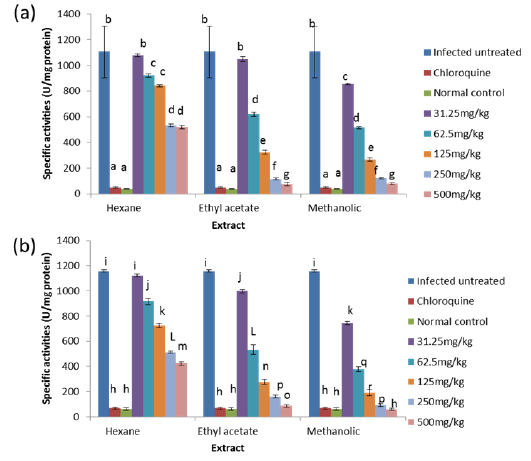
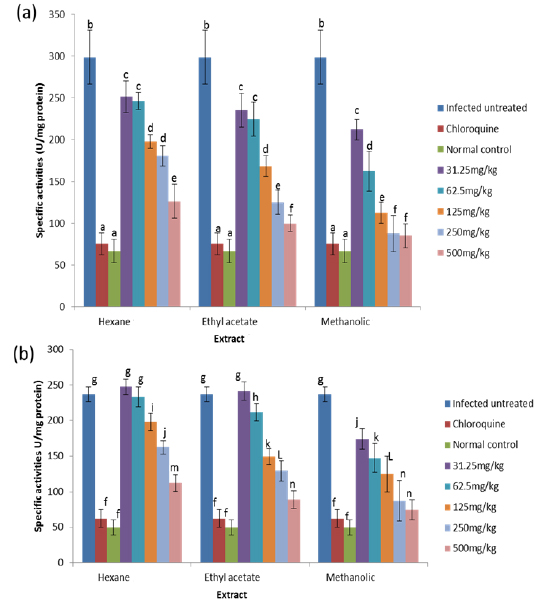
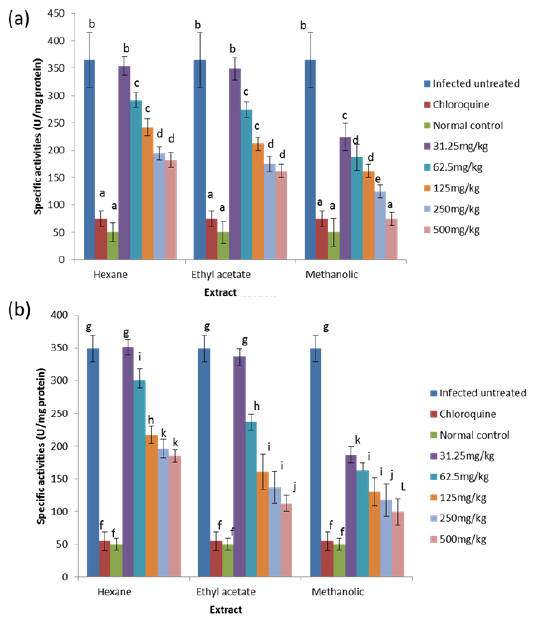
Effects on Antioxidant enzymes in vivo
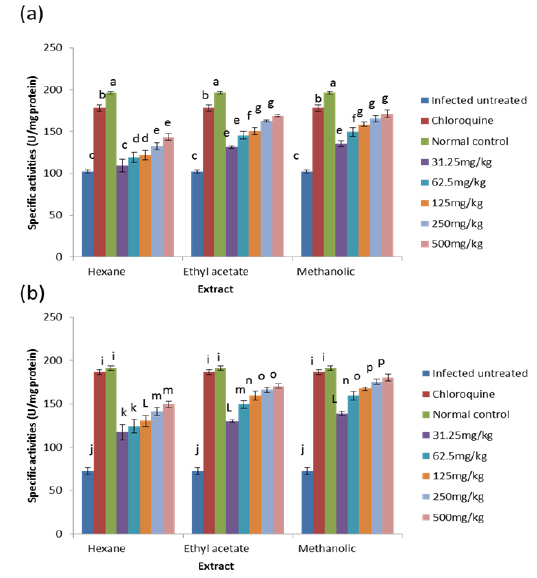
The results obtained showed that on day 4 post-inoculation, all the extracts at various doses significantly increased (
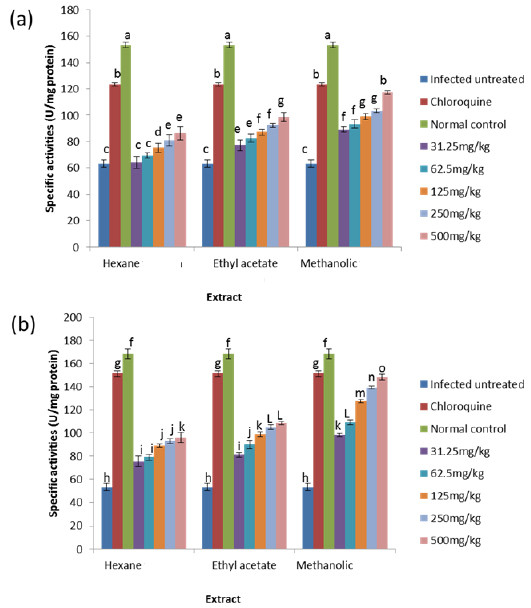
On day 4 post-inoculation, all the extracts at various doses significantly reduced (
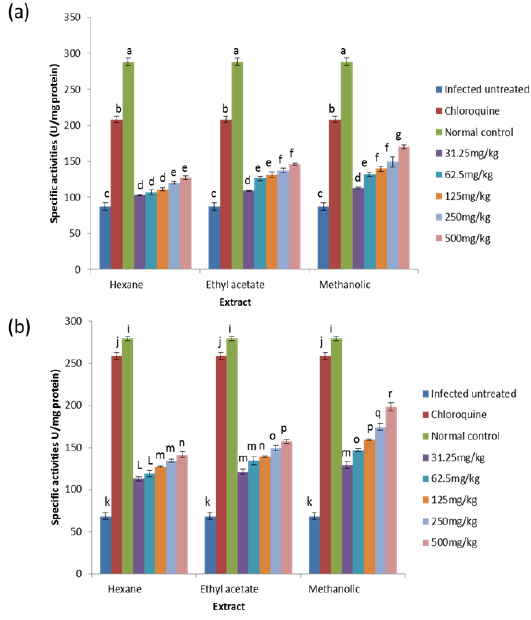
On day 8 post-inoculation, the activities of catalase in the erythrocytes and liver of infected animals were significantly increased (
Oxidative stress results from an imbalance between the generation of reactive oxygen species (ROS) and endogenous antioxidant systems (Chanda and Dave, 2009; Gyamfi et al., 1999). ROS are known to play a prominent role in the pathophysiology of malaria. A potent broad spectrum scavenger of these species may serve as a possible preventive intervention for free radical-mediated tissue damage associated with malaria (Ahmed et al., 1998).
From the results obtained in the in vitro studies, all the extracts exhibited DPPH, H2O2, and superoxide ion scavenging activities as well as the reducing effect on ferric ion, with the methanolic extract having consistent higher activity and comparing favourably with BHT used in the assays (Figs. 1 - 4). This may be due to the presence of phenolic compounds and flavonoids and the appreciable quantities of vitamins C, E, and selenium in the extracts.
Various studies have shown that a number of natural products including polyphenols, terpenes and various plant extracts exert antioxidant action (Chanda and Dave, 2009; Zhou and Zheng, 1991). Phenolic compounds are a class of antioxidant agents, the activity of which is mainly due to their redox properties which allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers (Hasan et al., 2009; Rice-Evans et al., 1997; Shahidi and Wanasundara, 1992). Phenolic compounds have been used to reverse, retard, or delay the onset of some diseases (Farombi and Owoeye, 2011). Flavonoids also have an established antioxidant activity (Cook and Samman, 1996; Farombi et al., 2002). The advantage of the antioxidant property of flavonoids has been revealed in neurotoxic studies which established that flavonoids can traverse the blood brain barrier (Youdim et al., 2003). Vitamin C is the major circulating water soluble antioxidant and acts as a free radical scavenger; it has been shown to take part in the metabolism of folic acid, red blood cell formation and maturation, bone formation and immune response mechanisms (De Tullio, 2010; Rao, 2006). Vitamin E is a lipid soluble vitamin and its main function is to prevent the peroxidation of membrane phospholipids and avoid cell damage through its antioxidant action (Herrera and Barbas, 2001; Padayatty et al., 2003). Selenium protects the cells by inhibiting free oxygen radical production but is best known for its role in glutathione enzyme system (Balakrishnan and Anuradha, 1998; Yalçin et al., 2003). Furthermore, vitamin E is transported by selenoproteins (Alfin-Slater and Morris, 1963). Thus, these antioxidant species in the extracts may play an important role in scavenging the ROS produced during Plasmodium species infection either independently or synergistically through mechanisms which may include interference with lipid peroxidation, which has been shown to occur in malaria (Kulkarni et al., 2003) and termination of free radical chain reactions (Sardesai, 1995). Furthermore, the higher activities obtained for the methanolic extract may result from the higher concentrations of these antioxidant species in this extract compared to other extracts. This implies that the methanolic extract of the leaf extract of
The results obtained for the in vivo antioxidant enzymes showed that treatment with the various extracts was able to increase the activities of SOD in the erythrocytes and liver which were reduced by infection (Figs. 5 - 8). SOD is a class of closely related enzymes that catalyze the breakdown of the superoxide anion into oxygen and hydrogen peroxide, thus protecting the cell from superoxide toxicity (Zelko et al., 2002). They are present in almost all aerobic cells and in extracellular fluids (Bannister et al., 1987). The observed decrease in the activities of both erythrocytic and hepatic SOD due to infection may be as a result of depletion owing to utilization of the enzyme to counteract oxidative damage (Hunt and Stocker, 1990).
Production of ROS species by the immune cells and the synchronized release of O2- during haemoglobin degradation by the malaria parasites might have contributed to the depletion of SOD possibly by overstressing the induction of its synthesis (Sohail et al., 2007). Furthermore, the antioxidant enzymes degraded by malaria parasites to derive amino acids cannot be replenished by the red blood cells due to lack of machinery for protein synthesis (Das and Nanda, 1999). However, the extracts.
CAT is an enzyme present in the peroxisomes of nearly all aerobic cells; it serves to protect the cell from the toxic effects of hydrogen peroxide by catalyzing its decomposition into molecular oxygen and water without the production of free radicals using either an iron or manganese co-factor (Chelikani et al., 2004). CAT and glutathione peroxidase are involved in the elimination of hydrogen peroxide although catalase has been regarded as a major determinant of hepatic antioxidant status (Mishra et al., 1994). It has been shown that trophozoite infected human red cells produce twice as much hydrogen peroxide and hydroxyl radicals as normal erythrocytes (Sohail et al., 2007). Increased intracellular iron levels from haemolysis can also lead to an increased production of hydrogen peroxide and hydroxyl ions (Vander et al., 1992). The observed significant decrease in the activity of catalase in both the erythrocytes and liver of infected animals may be due to depletion of catalase in combating oxidative stress produced by the infection. Decrease in the enzyme activities may also be due to utilization of erythrocytic proteins by the parasite. Hydrogen peroxide produced by the parasite during digestion of host cell cytosol also appears to be partially handled by host catalase (Becker et al., 2004). However, the extracts, most especially the methanolic extract, were able to ameliorate this reduction in CAT activities to a significant extent in the treated mice, possibly by sparing it because of their own inherent antioxidant composition.
GPx is the key enzyme responsible for the detoxification of cellular H2O2. It exists in two forms: Selenium dependent and Selenium independent. The former both detoxifies H2O2 and converts lipid hydroperoxides to non-toxic alcohols (Dreher et al., 1997); whereas, the latter enzyme is responsible for metabolizing lipid peroxides (Moon et al., 1983). All glutathione peroxidases catalyze the reduction of H2O2 using glutathione as substrate. Both GPx and catalase have the ability to inactivate intracellular H2O2, although GPx is considered the preferential pathway for elimination at low concentrations of H2O2 (Claudio et al., 2008; Jacob and Jandl, 1966). The observed increase in the activity of glutathione peroxidase may be due to an increased synthesis of the enzyme as a compensatory response to increased oxidative stress in both liver and blood of untreated infected animals. This observation agrees with that of Claudio et al. (2008) who reported an increase in the activity of glutathione peroxidase in the blood of patients with malaria. The increased activity may also be due to the fact that Plasmodium species produce glutathione peroxidase in response to reactive oxygen species formed during haemoglobin degradation (Atamna and Ginsburg, 1993).
GR is a flavoprotein that catalyzes the reduction of GSSG to the GSH which is an important cellular antioxidant (Muller, 2004). It is part of the glutathione enzyme system along with glutathione peroxidases and glutathione-S- transferase (Hayes et al., 2005). The observed significant increase in the activity of glutathione reductase in the untreated infected mice may be for the same reason as that of glutathione peroxidase because these two enzymes are closely linked in recycling glutathione (Postma et al., 1996). The observation that these two enzymes were reduced in a dose-dependent manner in both the blood and liver of animals treated with extracts, suggest an effective clearance of the free radicals generated as a result of the infection by the extracts. Moreover, since the extracts have the capacity of reducing parasitaemia in the infected animals (Balogun et al., unpublished data), the amount of free radicals generated will be reduced which may not necessitate increased synthesis of the enzymes, thus accounting for the reduction in activities of GPx and GR in a dose-dependent manner.
The results obtained in this study suggest that