Inflammation is a complex non-specific response of innate immunity, which is responsible for fighting against harmful stimulus, such as pathogens, damaged cells, and irritants (Ferrero-Miliani et al., 2007). Rheumatoid arthritis is a kind of common auto-immune disease of joint inflammatio n:the pathogenesis of this synovial inflammation occurs through the irreversible damage of articular cartilage and destruction of underlying joint (Grainger and Rowbotham, 2013;Tak and Bresnihan, 2000). During the inflammation on synovium, the articular structures are destroyed by the aggressive joint l ining, and the synovium is being infiltrated with T cells, B cells and macrophages. The inflammatory process of rheumatoid arthritis is initialized by stimulation of exogenous or endogenous antigens on synovial cells (Scott et al., 2010). Of which, macrophages are activated to overexpress pro-inflammatory cytokines, e.g. interleukin-6 (IL-6) and IL-1β; these cytokines elevate the catabolic process of cartilage cells via a NF-κB signaling pathway (Marok et al., 1996). The activation of catabolic cascade is able to induce the secretion of matrix metalloproteinases (MMPs ) and to degrade the extra-cellular matrix of cartilage (Bresnihan, 1999; Mix et al., 2001). In this scenario, the blockade of pro-inflammat ory cytokines is clinically relevant with potential targets fo r the therapy of rheumatoid arthritis (Feldmann and Maini, 2008).
Linderae Radix, the dry root of
Linderae Radix, the dry roots of
>
Extraction and isolation of herbal materials
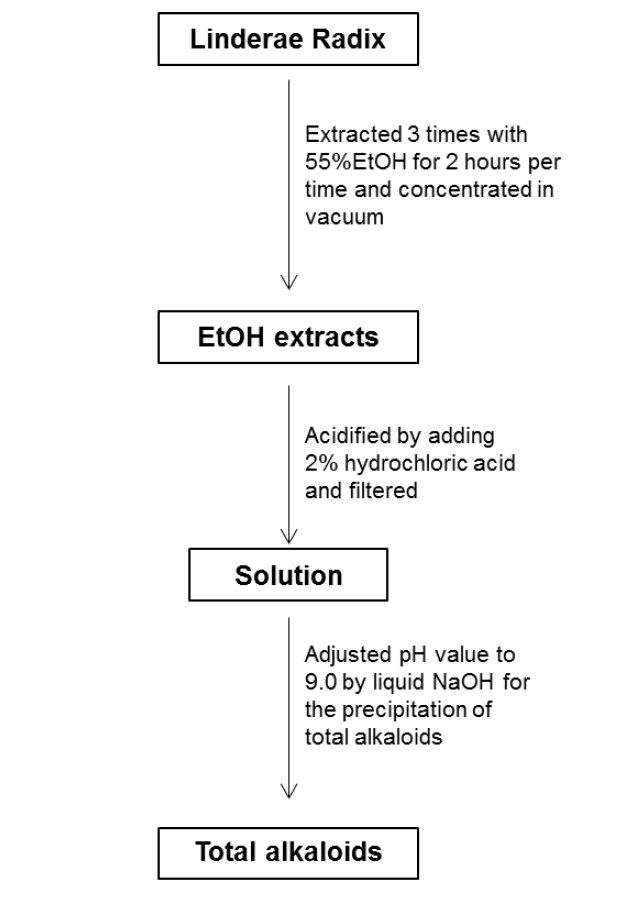
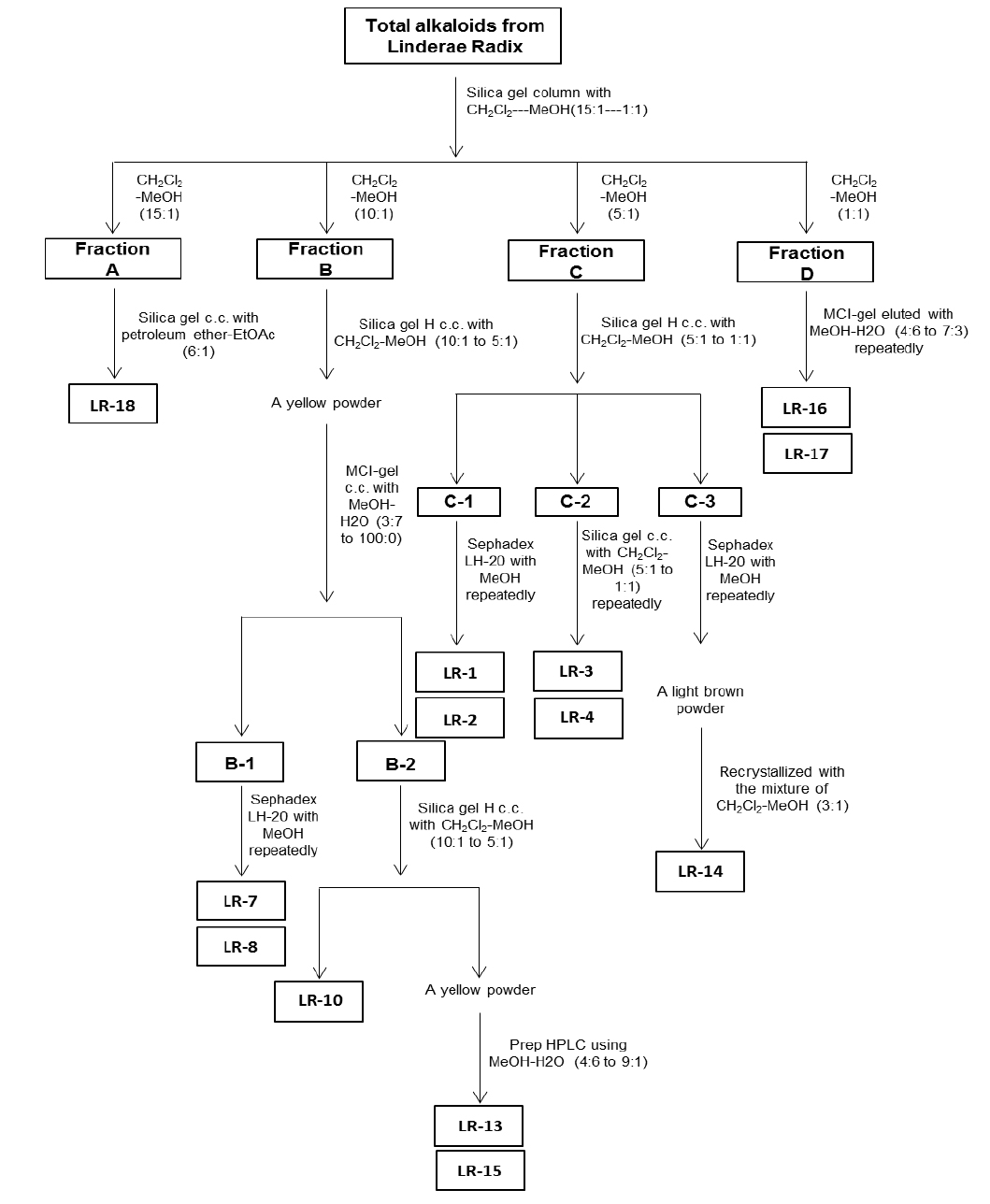
For the ethanol extraction, eight kg Linderae Radix powder were extracted by 55% ethanol, and refluxed for 2 hours, three times. The solvent was evaporated to give dark viscous extracts (Fig. 1S). Then, 2% hydrochloric acid was used for acid dissolution of alkaloids. The precipitation of total alkaloids was generated from alkaline precipitation by dropping 10% sodium hydroxide to adjust the pH value to 9.0 and lyophilizing at 0.02 mbar, -52℃. The powder of total alkaloids was then eluted with solvent system composed of dichloromethane and methanol in silica gel column to obtain four fractions (fraction A to D). Fraction A was subjected to silica gel column eluted with petroleum ether and ethyl acetate (6:1) to yield linderane; Fraction B was subjected to silica gel high performance column eluted with dichloromethane and methanol (10:1 to 5:1), then separated by MCI-gel CHP 20P column eluted with methanol and water (3:7 to 100:0) to generate 2 fractions, B-1 and B-2. B-1 was purified by Sephadex LH-20 column with methanol to yield isoboldine and reticuline (Xiao et al., 2004; Yu et al., 2003). And B-2 was chromatographed by silica gel high performance column eluted with dichloromethane and methanol (10:1 to 5:1) to form N-methyllaurotetanine (Zhu et al., 2007). The yellow powder was then purified by prep HPLC on YMC RP-18 column eluted with methanol and water (4:6 to 9:1) to give actinodaphnine and pallidine (Guinaudenu et al., 1979; Xiao et al., 2006); Fraction C was eluted by silica gel high performance column with dichloromethane and methanol (5:1 to 1:1) to give 3 fractions, C-1, C-2 and C-3. C-1 was then applied to Sephadex LH-20 column with methanol repeatedly to yield norisoboldine and lindcarpine (Rachmatiah et al., 2009). C-2 was subjected to silica gel column eluted with dichloromethane and methanol (5:1 to 1:1) to yield boldine and linderaline (Chou et al., 2005; Xiao et al., 2004). C-3 was purified by Sephadex LH-20 column with methanol and recrystallized with dichloromethane and methanol (3:1) to generate norjuziphine (El-Kawi et al., 1984); Fraction D was then decolorized and eluted by MCI-gel column with methanol and water (4:6 to 7:3) to give secoboldine and secolaurolitsine (Chiou et al., 1998; Milian et al., 2004) (Fig. 2S).
RAW 264.7, mouse macrophage cell line from American Type Culture Collection (ATCC, Manassas, VA), was maintained in culturing DMEM (Invitrogen, Carlsbad, CA) supplemented with 5% FBS (Invitrogen), 100 g/ml penicillin and 100 g/ml streptomycin (Invitrogen), at 37℃ in a water saturated 5% CO2 incubator. The viability of macrophage RAW 264.7 cells was measured by MTT assay. Cells were cultured in 96-well plate. After the treatment of isoquinoline alkaloids for 24 hours, MTT (0.5 mg/ml) was added into the cultures, then extracted by DMSO solvent. The absorbance at 570 nm was measured. The cell viability was calculated as the percentage of the absorbance value of negative control while the value of negative control was 100%.
>
Quantification of the mRNA expressions
Macrophage RAW 264.7 cells were cultured in 6-well plate at a number of 2 × 105 cells per well in 5% CO2, 37℃ atmosphere. After incubation for 24 hours, each isoquinoline alkaloid was pre-treated. And 3 hours later, LPS, the major ingredient on the membrane of Gram negative bacteria binding to Toll-like receptor-4 (TLR-4) on macrophage and promoting the gene expression of pro-inflammatory cytokines, was applied onto the cultures for 20 hours. Then, the cultured medium was discarded, and cells were collected and stored at -80oC overnight (Du et al., 2013). The total RNA was isolated with RNAzol reagent (Molecule Research Center, Cincinnati, OH) according to the manufacturer’s protocol. Three μg total RNA extracted from cultured cells were then reversely transcribed by Moloney Murine Leukemia Virus (MMLV) reverse transcriptase kit (Invitrogen) with oligo-d(T) primer (Invitrogen) in a 20 μl reaction. And quantitative real-time PCR was performed by Faststart Universal SYBR Green Master (ROX) (Roche Applied Science, Mannheim, Germany), according to the manufacturer’s instructions. SYBR green signal was detected by 7500 Fast Real-Time PCR machine (Applied Biosystems, Brooklyn, NY). Calculation was done by using the Ct value of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to normalize the Ct value of target gene in each sample to obtain first, which then deducted the ΔCt value of negative control to achieve the ΔΔ Ct value. (Winer et al., 1999). Transcription levels were then quantified by the percentage of activation compared with mRNA expression of LPS stimulation. PCR products were analyzed by gel electrophoresis on a 1.5% agarose gel, and the specificity of amplification was confirmed by melting curves. Primers employed in quantitative real-time PCR analysis were: 5’- GGA GTA CCA TAG CTA CCT GG-3’ and 5’- CTA GGT TTG CCG AGT AGA TC-3’ for murine IL-6 (283 bp; NM_031168); 5’- AAA TAC CTG TGG CCT TG-3’ and 5’-TTA GGA AGA CAC GGA TTC-3’ for murine IL-1β (296 bp; NM_008361); 5’-AGT GAC AAG CCT GTA GCC-3’ and 5’-AGG TTG ACT TTC TCC TGG-3’ for murine TNF-α (251 bp; NM_013693); 5’- AAC GGA TTT GGC CGT ATT GG-3’ and 5’- CTT CCC GTT CAG CTC TGG G-3’ for GAPDH (516 bp; L29427) used as internal control in all cases.
Statistical analysis (SPSS) was used to analyze the correlation of different bioactivity of isoquinoline alkaloids. Statistical tests were done by using one-way ANOVA. The data was separated into two groups to compare both the activation of tested compounds and control. The control group was varied in different experiments, and which was specified in the figure legends. Data were expressed as Means ± SD, where
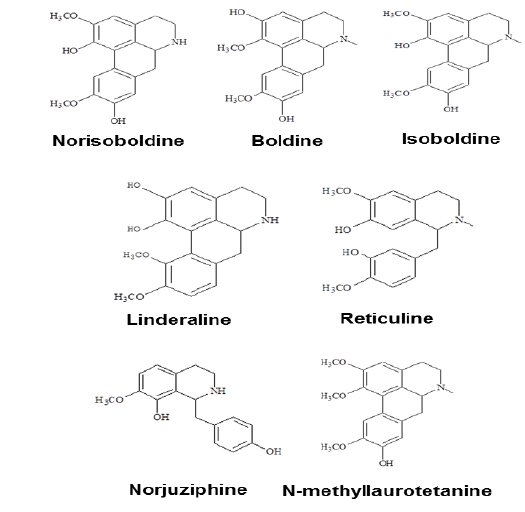
Linderae Radix (~ 8 kg) was powdered and then extractedby methods, shown in Supporting information 1 (Fig. 1S).After lyophilization, the resulting precipitate of total alkaloids from Linderae Radix was ~ 200 g. Then, the total alkaloids were subjected to column chromatography by different solvents and elution, which was not described fully in here. The purification steps were summarized in Fig. 2S. The chemical structures for products with higher isolation yield including norisoboldine, boldine, linderaline, isoboldine, reticuline, N-methyllaurotetanine, norjuziphine, were spectrally analyzed by 1H and 13C-NMR, shown in Fig. 1 and Appendix.
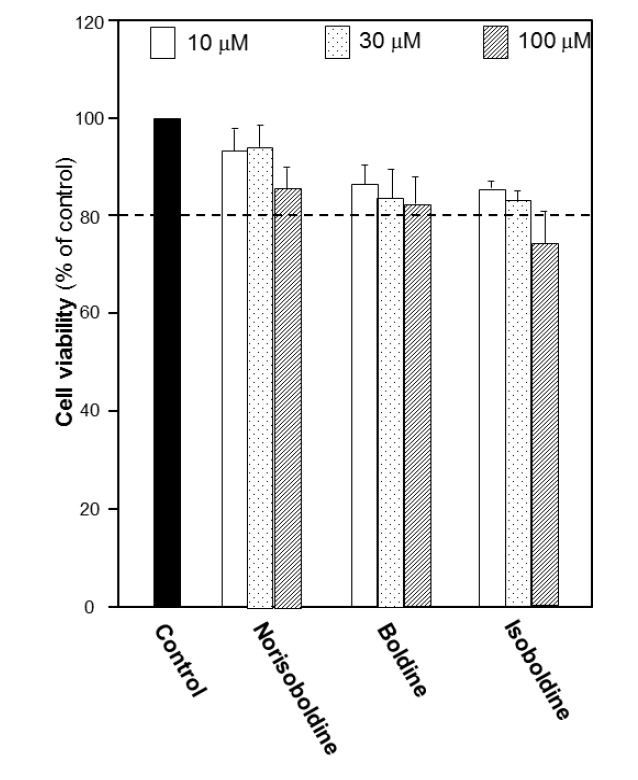
The growth of macrophage RAW 264.7 cells was explored to determine the cell number used in the following experiments. The number of 10,000 cells per well was chosen to detect the viability under the treatment of the alkaloids. Before screening for bioactivities of alkaloids, the cytotoxicity of each alkaloid in RAW 264.7 cells was first performed by MTT assay in different concentrations. The results suggested that for each isoquinoline alkaloid, the doses below 30 μM did not show remarkable cytotoxicity or proliferation effect. At 100 μM, the cytotoxicity was found. Thus, 30 μM was recognized as the most applicable working concentration for alkaloids in the following experiments (Fig. 3S), which provided a critical reference on choosing the concentration of alkaloids.
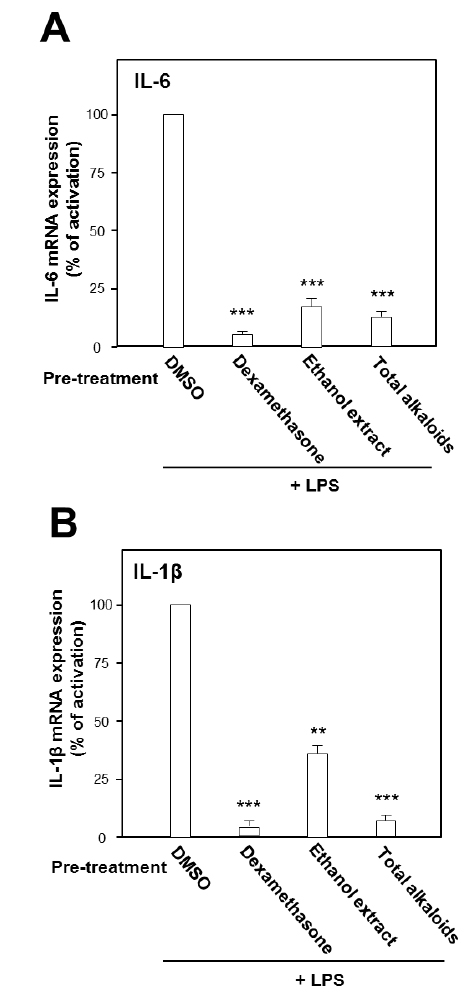
Under the stimulation of LPS, the mRNA expressions of pro-inflammatory cytokines were measured by real-time PCR. LPS at concentration of 0.1 μg/ml was selected as the concentration for treatment of herbal extract and alkaloids. The ethanol extract, total alkaloids and seven alkaloids were applied onto the cultured cells. As shown in Fig. 2, the ethanol extract and the total alkaloids could substantially suppress the gene transcription levels of pro-inflammatory cytokines, IL-6 and IL-1β, stimulated by LPS. The suppression on both cytokines was more robust in the treatment of total alkaloids than that of the ethanol extract, which could be revealed by over 80% of suppression (Fig. 2).
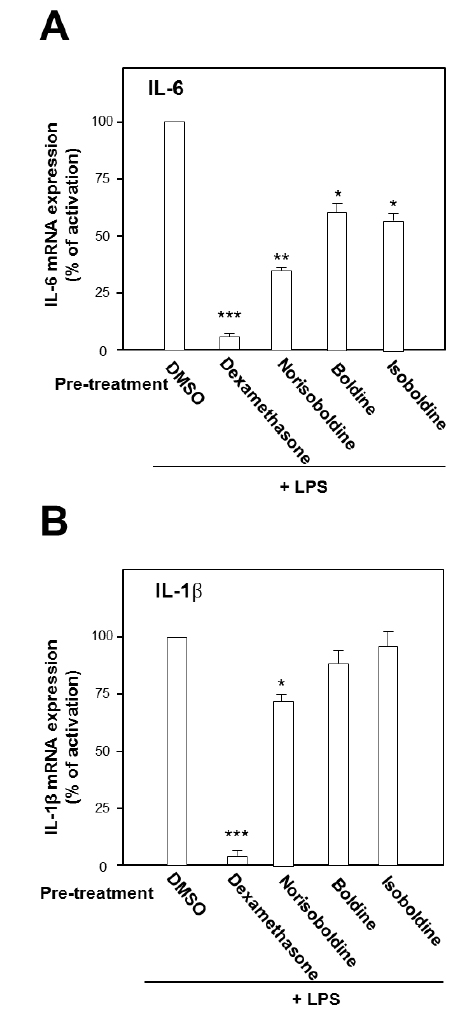
The role of individual alkaloid was tested in the cell model. At the largest working concentration (30 μM), norisoboldine, boldine and isoboldine significantly reduced theLPS-enhanced mRNA expression of IL-6 (Fig. 3A). In addition,the expression of IL-1β, induced by LPS, was suppressed by both applications of boldine and norisoboldine at 30 μM (Fig. 3B). The other alkaloids did not show this suppression (data not shown). These results indicated that the ethanol extract, total alkaloids and few isoquinoline alkaloids from Linderae Radix showed inhibition against the LPS-enhanced gene expressions of pro-inflammatory cytokines, IL-6 and IL-β, on mRNA levels. By comparing to the total alkaloids, individual alkaloids exerted limited or little effect on reducing gene transcription levels of IL-6 and IL-1β up-regulated by LPS, which suggested a possible synergistic effect of different alkaloids within Linderae Radix.
Rheumatoid arthritis is caused by a cascade of auto-immune disorder, which could induce cartilage damage and bone destruction. Owing to expensive costs of physical therapy and side effects of synthetic drug, the development of a cost-effective and safe treatment of rheumatoid arthritis has been searched for many years. TCM has been well documented for their fewer side effects, and Chinese are very keen on this beneficial advantage for health care of various diseases. Therefore, herbal medicine might support a novel direction for the treatment of rheumatoid arthritis. Linderae Radix, a TCM used for alleviating pain and nourishing organs, was studied here for the purpose of a possible development of a drug candidate in treating rheumatoid arthritis. In LPS – induced RAW 264.7 cells, the ethanol extract and total alkaloids could dramatically decrease the enhanced mRNA expression of IL-6 and IL-1β. In addition, the isoquinoline alakloids, including norisoboldine, boldine and isoboldine, displayed their inhibition on the LPS – stimulated mRNA levels of those proinflammatory cytokines.
Norisoboldine, boldine and isoboldine might be the effective components in alkaloids deriving from Linderae Radix. The findings indicated that both herbal extract and alkaloids from Linderae Radix might inhibit the LPS-induced IL-6 and IL-1β by blocking the phosphorylation of NF-κB subunits. As a result, the gene expression of pro-inflammatory mediators would be reduced, which would then prohibit the catabolic process of chondrocytes to produce MMPs. Thus, the reduction of destroying cartilage matrix caused by MMPs would potentially facilitate the therapy of rheumatoid arthritis.
However, the ethanol extract and total alkaloids showed better effect in the down-regulation of pro-inflammatory cytokines stimulated by LPS, suggesting a possible synergistic role of different alkaloids. Furthermore, other alkaloids that had not been isolated yet might exist in the herbal medicine with anti-inflammatory effects via suppressing LPS-enhanced cytokines. Nevertheless, this study provided a plausible explanation for the therapeutic potency of alkaloids from Linderae Radix on anti-rheumatoid arthritis.
Total alkaloids and norisoboldine, derived from Linderae Radix, were demonstrated to prevent the production of LPS-induced mediators of inflammation in RAW 264.7 cells dose-dependently by the suppression on NF-κB and MAPKs signaling pathways (Luo et al., 2009; Luo et al., 2010). Our results are in line to the previous results that the addition of boldine and isoboldine were considered as the active ingredient of Linderae Radix. The amount of norisoboldine (~ 1% of total alkaloids) in the herb is much higher than that of boldine and isoboldine (~ 0.05% and ~ 0.035%, respectively of total alkaloids). Under this scenario, norisoboldine should be the key alkaloid in triggering this cytokine suppression. Thus, Linderae Radix or its alkaloids could be further developed as the possible candidates of therapeutic agents against rheumatoid arthritis.