



Polyoxometalates (POMs), anionic early transition metal oxide clusters, are attractive inorganic building blocks owing to their distinctive structures and excellent catalytic properties, which lead to a vast range of applications.1,2 One of the fundamental aspects that bother POM chemists is their limited stability in aqueous solutions (at nearly neutral or basic pH) which tremendously narrows the scope of their application as catalysts3 or antiviral and antitumoral drugs4 in the chemical or biological systems. Although organic solvents can effectively protect POM skeleton from degradation and aggregation,5 they are strictly used in the synthetic biomimetic systems.6 Therefore finding an alternative to replace organic solvents is rather demanding for both synthetics and application purposes.
Cyclodextrins (CDs), torus-like macro-rings built up from glucopyranose units and known to form non-covalent complexes with many organic compounds,7,8 are found to have promising applications in the area of POM chemistry.9-15 For example, Proust et al.14 showed the enhanced stability of a POM–organotin hybrid, a Dawsontype K7[a2-P2W17O61 {Sn(C6H4I)}] (1) in basic media (under which hybrid 1 instantly dissociated into its monolacunary precursor [P2W17O61]10- in the absence of β-CD) due to the formation of water-soluble complexes with β-CD (β-CD⊃1). g et al.15 used vanadium-substituted heteropoly acids/cyclodextrin complexes (PMoVn-β-CD, n = 1, 2) as phase transfer catalysts in direct hydroxylation of benzene to phenol. However, in-depth investigation on the structural feature of POM/CD complexes is lacking.
In this study, we employed ESI-MS to present how cyclodextrin can dramatically improve the stability of Keggin phosphotungstic acid (formulated as H3PW12O40) in both aqueous phase and gas phase via complexation with g-CD. In addition, the influence of different types of solvents on the stability of Keggin H3PW12O40 was also addressed firstly by ESI-MS.
Sample preparation. All reagents were purchased from commercial sources and used without further purification. Solutions were prepared at concentrations of approximately 10-5 M using ultra-purified solvent.
Mass spectrometry. Mass spectrometric experiments were performed using an Agilent 6520 Q-TOF LC/MS mass spectrometer in the negative ion mode. The dualspray electrospray ionization source condition was as follows: Vcap 3500 V; skimmer 65 V; drying and nebulizer gas N2 ; nebulizer 30 psi; drying gas flow 9 L/min; drying gas temperature 330 ℃ fragmentor 100 V; scan range 50-3000 m/z; injection volume 0.5 mL. The samples were transferred to the electrospray source via an autosampler with a flow rate of 0.2 mL/min. CID experiments were performed using N2 as the target gas. Each experiment was repeated at least three times under the same experimental conditions.
>
How solvents and γ-CD affect the solution stability of H3PW12O40
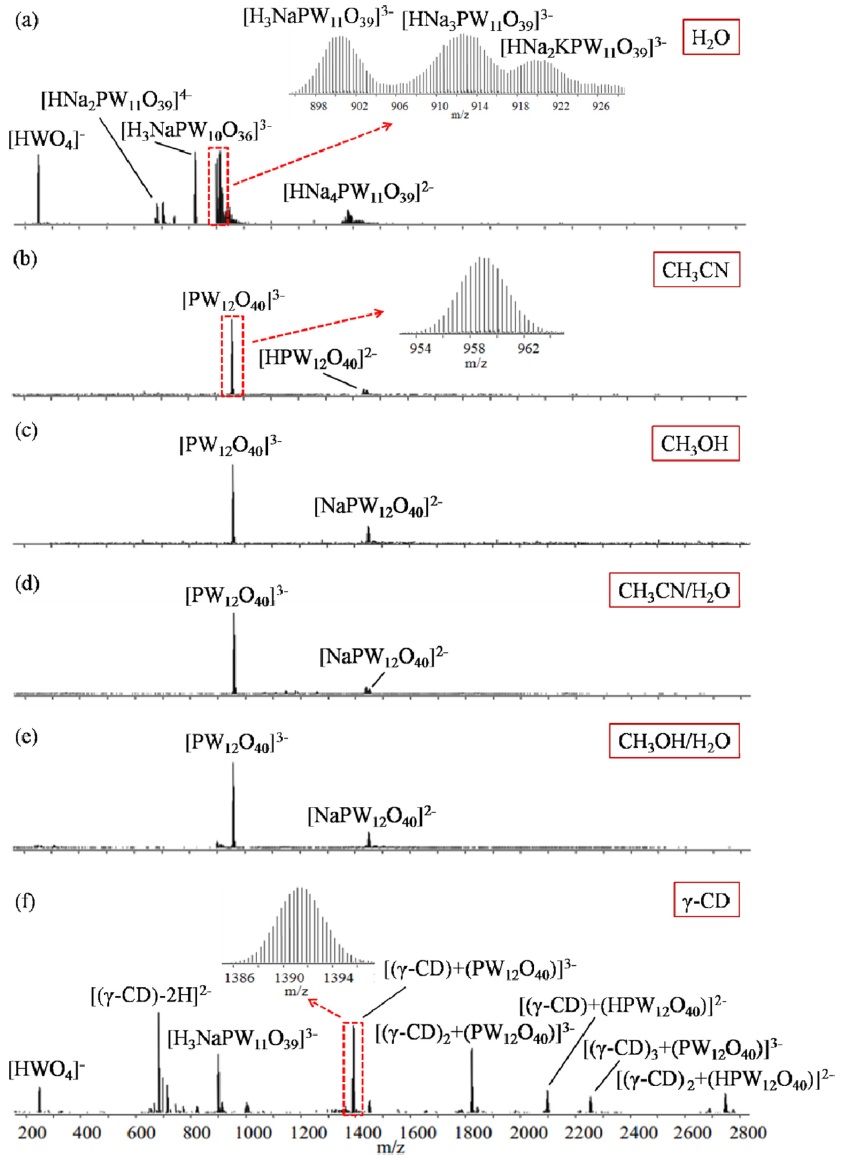
We choose H2O, CH3CN, CH3OH, CH3CN/H2O and CH3OH/H2O as different types of solvents to see if the POM can keep its structural integrity in such solutions (Figs. 1(a)-1(e)). Fig. 1(a) shows that H3PW12O40 can hardly maintain its structural integrity in pure H2O and dissociates completely into the mono-, dilacunary anions (W11 and W10) and even the monomeric species (W1) with a high degree of cationization, indicated by peaks for [HNa2PW11O39]4- (m/z 683, 48%), [H3NaPW11O39]3- (m/z 901, 78%), [HNa3PW11O39]3- (m/z 912, 79%), [HNa2KPW11O39]3- (m/z 920, 46%), [HNa4PW11O39]2- (m/z 1380, 10%), [H3NaPW10O36]3- (m/z 823, 100%) and [HWO4]- (m/z 249, 75%). This is a very important experimental evidence which implies that the composition of POM catalyst may change during the reaction course when the reaction media is pure water.
However, the poor stability of H3PW12O40 can be greatly improved by using pure organic solvents or even mixed organic/water solvents. Figs. 1(b)-1(e) show the existence of intact polyoxoanion of [PW12O40]3- at
Is it possible to find an alternative in lieu of organic solvents to some extent in order to stabilize the fragile POM’s skeleton in pure H2O? Fig. 1(f) is the ESI-MS spectrum of an equimolar mixture of H3PW12O40 and γ-CD in pure H2O (H3PW12O40 dissolved in an aqueous solution of γ-CD). It can be clearly seen that the intact polyoxoanion [PW12O40]3- can be mostly retained by forming a series of stable non-covalent complexes with γ-CD, [(γ-CD) + PW12O40]3- (m/z 1391, 80%), [(γ-CD)2 + PW12O40]3- (m/z 1823, 60%), [(γ-CD)3 + PW12O40]3- (m/z 2256, 15%), although there are still some fragments of H3PW12O40 such as [H3NaPW11 O39]3- and [HWO4]-. The interactions between PW12O40]3- and γ-CD were anticipated to be hydrogen bond (H-bond) and ion/dipole interactions due to the ample hydroxyl groups on the outer rims of γ-CD serving as Hdonors to bind with the terminal and/or bridging oxygen atoms (H-acceptors) of [PW12O40]3-. This will provide a valuable methodology for maintaining polyoxoanions intact in solution either by a selection of organic solvents or organic additives.
The instrument parameters in particular the fragmentor voltage, which is the difference between the capillary and skimmer potentials in the source-analyzer interface region of the mass spectrometer, play a key role on the detection of POM-based non-covalent complexes in the gas phase. Increasing the fragmentor voltage from 120 to 180 V led to a dissociation of the {PW12 }/γ-CD complexes into lownuclearity species (e.g. W4 and W5). The fragmentor voltages were chosen to maximize gas-phase yields and minimize decompositions of the target analyte.
>
The gas-phase fragmentations of non-covalent {PW12}/γ-CD complexes
As defined in Ma’s work16 as well as our previous paper17 that a common fragmentation feature for the bare polyoxoanion [PW12O40]3- is to yield pairs of complementary product anions as a result of cleavages of multiple W-O and P-O bonds of the anion, each pair comprising a dianionic isopolyoxometalate [WxO3x+1]2- and dianionic heteropolyoxometalate [PW12-xO39-3x]-. However, when this bare polyoxoanion form non-covalent complexes with γ-CD, their dissociation pathways are completely different (Fig. 2). It is shown in the CID mass spectrum of 1:1 {PW12}/γ-CD complex, [PW12O40 + γ-CD]3-, that an intermolecular hydrogen -transfer reaction occurred between [PW12O40]3- and γ-CD hich resulted in the complementary anionic pairs, deprotonated [γ-CD - H]- and protonated [HPW12O40]2-. The proton-transfer reaction taken place between [PW12O 40]3- and γ-CD is supposed to be driven by the strong tendency for the polyoxoanion to abstract a proton onto the bridging oxygen atoms of the cluster framework, which consequently reduces the net charge of the bare polyoxoanion, and the release of coulombic repulsions in the generation of the two resultant products. In contrast to breaking of multiple W(P)-O bonds of the bare cluster [PW12O40 ]3-, fragmentation of the 1:1 {PW12}/γ-CD complex do not show any of fragments related to the POM skeleton rupture, in other words, γ-CD can effectively protect [PW12O40]3- from decomposition in the gas phase. For the 1:2 and 1:3 {PW12}/γ-CD complexes, γ-CD was lost as an neutral species in the gas-phase dissociations. The distinctive fragmentation pathways of {PW12}/γ-CD complexes from [PW12O40]3- unveil the role of γ-CD on the stabilization of the highly negative-charged POM in the gas phase.
In summary, this study demonstrated the stabilizing role of a cyclodextrin on Keggin [PW12O40]3- via H-bonding complexation by ESI-MS. The different fragmentation pathways of the {PW12}/γ-CD complexes versus [PW12O40]3- showed that the so-called “weak” non-covalent interactions can effectively change the dissociation chemistry of POM in the gas phase. Also, the influence of different types of solvents on the solution stability of H3PW12O40 was presented by ESI-MS. The poor stability of H3PW12O40 in pure H2O can be greatly improved either by dissolving it in pure organic or mixed organic/water solvent or by addition of γ-CD in pure H2O. The latter strategy provides a valuable alternative in addition to organic solvents to keep the polyoxoanion intact in solution. Moreover, the synergy with respect to structure and catalytic property will bring the POM/CD complexes into more fascinating applications particularly as (inorganic-organic) hybrid catalysts. Further study on the enhanced catalytic activity of {PW12}/γ-CD is in progress.
![CID mass spectra of (a) [PW12O40]3- ; (b) [(γ-CD)+(PW12O40 )]3- ; (c) [(γ-CD)2 +(PW12O40)]3- ; (d) [(γ-CD)3 +(PW12O40)]3-. The parent ion (denoted by a diamond) was shown in a red square box in each spectrum.](http://oak.go.kr/repository/journal/16999/E1MPSV_2015_v6n1_13_f002.jpg)