Marine algae generate an abundance of active substances that have potential in the pharmaceutical and biomedical industries (Kang et al. 2012). Recently, many researchers have provided evidence that algae exhibit various biological effects both
Carotenoids, a family of more than 700 pigments, are naturally occurring tetraterpenes found in various fruits, vegetables, plants, algae and bacteria (Kim et al. 2010, Sivathanu and Palaniswamy 2012). They have recently attracted interest not only as a source of pigmentation but also for their beneficial effects on human health (Heo et al. 2012). For example, fucoxanthin, mainly present in brown algae, is along with carotene, one of the most abundant carotenoids found in nature (Hosokawa et al. 2004, Heo and Jeon 2009, Fung et al. 2013, Kim et al. 2013). Fucoxanthin is one of the major xanthophylls, and contributes more than 10% of the estimated total production of carotenoids in nature, especially in the marine environment. In Southeast Asia, several types of brown algae that contain fucoxanthin are often consumed particularly in soups (Maeda et al. 2008). Many of the biological functions of fucoxanthin have been previously characterized, including anti-oxidant, anti-tumor, anti-inflammatory, and anti-obesity effects (Hosokawa et al. 2004, Heo et al. 2008, Kim et al. 2010, Kang et al. 2011, Yu et al. 2011, Heo et al. 2012, Kim et al. 2013). However, information concerning its ability to inhibit matrix metalloproteinases (MMPs) in human fibrosarcoma is limited.
MMPs digest components of the extracellular matrix, such as fibrillar and non-fibrillar collagens, fibronectin, laminin, elastin and basement membrane glycoproteins under physiological conditions (Kim et al. 2006, Debret et al. 2008, Park and Kim 2012). Inhibition of MMPs is possible at several biochemical sites and a number of MMP inhibitors are being identified as therapeutic agents for cancer. Gelatinases are secreted as inactive zymogens, with cleavage of a prodomain yielding the active form. Both MMP-2 and MMP-9 can degrade type IV collagen of 356basement membranes, which are the first barriers against cancer invasion (Kong et al. 2010, Bauvois 2012). Elevated MMP-9 and MMP-2 levels in fibrosarcoma cells result in markedly enhanced invasive tumor growth (Kong et al. 2008). Thus, the inhibition of MMPs produced by cancer cells is believed to be crucial in delaying cancer invasion.
The objectives of the current study were to isolate 9′-
The marine
Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin and trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA) were obtained from Gibco BRL, Life Technologies (Grand Island, NY, USA). Primary and secondary antibodies used for Western blot analysis were MMP-2 (sc-13595), MMP-9 (sc-10737), tissue inhibitor of metalloproteinase (TIMP)-1 (sc-21734), nuclear factor кB (NF-κB) p65 (sc-8008), c-Jun N-terminal kinase (JNK) (sc-7345), p-JNK (sc-6254), p38α/β (sc-7149), p-p38 (sc-7973), β-actin (sc-130656), lamin B1 (sc-20682), goat anti-rabbit IgG-HRP (sc-2004), and goat anti-mouse IgG1-HRP (sc-2060), and purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Gelatin (type A) and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals and reagents used were of analytical grade.
>
Isolation and structural identification of compounds
Human fibrosarcoma HT1080 cells were obtained from American Type of Culture Collection (Rockville, VA, USA). Human fibrosarcoma HT1080 cells were grown in DMEM supplemented with 10% heat-inactivated FBS, 100 μg mL−1 of streptomycin and 100 U mL−1 of penicillin. The cells were incubated in an atmosphere of 5% CO2 at 37℃ and were sub-cultured every 3 days.
Cell viability was determined by MTT reduction assay as described by Hansen et al. (1989). HT1080 cells were seeded in a 96-well plate at a density of 1 × 104 cells well−1. After 18 h, cells were exposed with compounds at difference concentrations, and then the cells were incubated for 24 h at 37℃. MTT stock solution (50 μL) was then added to each well to a total reaction volume of 250 μL. After 4 h of incubation, the plates were centrifuged (800 ×g, 5 min), and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 μL of DMSO, and the absorbance was measured with an enzyme- linked immunosorbent assay plate reader (BioTek Instruments, Winooski, VT, USA) at 540 nm. Relative cell viability was evaluated in accordance with the quantity of MTT converted to the insoluble formazan salt. The optical density of the formazan generated in the untreated cells was considered to represent 100% viability. The data are expressed as mean percentage of the viable cells versus the respective untreated cells.
HT1080 cells were seeded in a 6-well plate at a density of 1 × 104 cells well−1. At 18 h after seeding, cells were pretreated with compounds at difference concentrations for 1 h before an injury line was made with a 2-mm width tip on cells that were plated in culture dishes at 80% confluence. After being rinsed with phosphate buffered saline (PBS) the cells were allowed to migrate in complete medium in the presence of compounds, and photographs were taken in series at time points.
>
Determination of MMP-2 and MMP-9 activity by gelatin zymography
The enzymatic activities of MMP-2 and MMP-9 in HT1080 cells treated with compounds were determined secby gelatin zymography. HT1080 cells in serum-free medium were seeded and pre-treated with different concentration s of compounds for 1 h. Expression of MMPs was stimulated by treatment with 10 ng mL−1 of PMA and the cells were incubated for 24 h. After incubation, conditioned media were collected and their protein contents were determined by the bicinchoninic acid (BCA) protein assay kit. Conditioned medium was electrophoresed under non-reducing conditions and without heating through a 10% sodium dodecyl sulphate (SDS)-polyacrylamide gels impregnated with 0.15% of gelatin. After electrophoresis, the gels were washed with 2.5% Triton X-100 solution at room temperature to remove SDS. Gels were incubated overnight at 37℃ in a developing buffer containing 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 5 mM CaCl2, and 0.02% Brif-35. The gels were stained with 1% Coomassie blue R-250 in 45% methanol and 10% glacial acetic acid, and destained in the same solution without the Coomassie blue dye. Gelatinolytic bands were observed as clear zones against the background stain of undigested substrate in the location of gelatinase. The intensity of the bands was estimated using Zoom Browser EX software (Canon USA Inc., Lake Success, NY, USA).
>
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from HT1080 cells treated with PMA in the presence or absence of compounds using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Equal amount of RNA was used for each cDNA synthesis reaction. Adjusted oligo dT primer (10 μM) with DEPC was added and then incubated for 5 min at 70℃ and was then snap-cooled on ice. With isolated mRNA were syntheses to cDNA following to manufactor’s instruction (Promega). Polymerase chain reaction (PCR) was carried out in an automatic Whatman thermocycler (Biometra, Kent, UK). Single stranded cDNA was amplified by PCR with specific primers. Primer sequences used to amplify the desired cDNA fragment were as follows: MMP-2 (forward primer, 5′-TGAAGGTCGGTGTGAACGGA-3′; reverse primer, 5′-CATGTAGCCATGAGGTCCACCAC-3′), MMP-9 (forward primer, 5′-CACTGTCCACCCCTCAGAGC-3′; reverse primer, 5′-CACTTGTCGGCGATAAGG-3′), TIMP-1 (forward primer, 5′-AATTCCGACCTCGTCATCAG-3′; reverse primer, 5′-TGCAGTTTTCCAGCAATGAG-3′), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward primer, 5′-GAAGGTCGGAGTCAACGGATTT-3′; reverse primer, 5′-ATGGGTGGAATCATATTGGAAC-3′) (Hanigan et al. 2008, Kong et al. 2010). The level of MMP-2, MMP-9, and TIMP-1 mRNA in the stimulated groups was normalized by the GAPDH mRNA level and compared to the mRNA level of control cells. The amplification cycles were carried out at 95℃ for 45 s, 56-62℃ for 1 min, and 72℃ for 45 s. After 30 cycles, the PCR products were separated by electrophoresis on 1.5% agarose gel for 20 min at 100 V. Gels were then stained with 0.01% ethidium bromide (No. C-9009; Bioneer, Daejeon, Korea) visualised by UV light using Gelmanager gel image analysis Zoom Browser EX software (Canon USA Inc.).
>
Protein extraction and Western blot analysis
HT1080 ells were lysed in lysis buffer (20 mM Tris, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP-40, 10 mg mL−1 aprotinin, 10 mg mL−1 leupeptin, and 1 mM phenylmethanesulfonylfluoride) for 60 min and then centrifuged at 12,000 rpm for 15 min at 4℃. For separate extraction of nuclear and cytoplasmic proteins, CelLytic NuCLEAR Extraction kit (Sigma-Aldrich Co., St. Louis, MO, USA) was used following manufacturer’s instructions, and protein concentration of cell lysates were determined by the BCA protein assay kit. Equal amounts of protein (30 μg) were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The resolved proteins were transferred onto a polyvinylidenedifluoride (PVDF) membranes (Millipore, Billerica, MA, USA) membrane from the gel and PVDF membranes blocked in 5% non fat dry milk in TBS-T (25 mM Tris-HCl, 137 mM NaCl, 2.65 mM KCl, 0.05% Tween 20, pH 7.4) for 2 h. The primary antibodies were used at a 1 : 500 dilution. Membranes incubated with the primary antibodies at 4℃ overnight. Thereafter, the membranes were washed with TBS-T and then incubated with the secondary antibodies used at 1 : 5,000 dilutions. Signals were developed using an ECL western blotting detection kit and quantified by LAS-4000 imaging system (Fujifilm, Tokyo, Japan).
The cells were culture and washed three times with PBS and fixed using a solution of 4% paraformaldehyde at room temperature for 15 min. After that, the fixed cells were permeabilized with TBS-T buffer at room temperature for 30 min. For immunofluorescence staining of NF-κB (p65) proteins, the cells were then incubated with primary antibodies against mouse NF-κB (p65) at room temperature and a dilution of 1 : 200 for 2 h. Cells were washed with TBS-T buffer, and then reacted with Alexa Fluor 546 Goat Anti-Mouse IgG (H+L)-conjugated secondary antibodies at room temperature for 1 h. Photomicrographs were obtained using by fluorescent microscopy (Carl Zeiss MicroImaging GmbH, Goettingen, Germany) systems. The yellow form of p65 is excited at 556 nm and emits at 573 nm. The nuclei were stained with 100 ng mL−1 of 4,6-diamidino-2-phenylindole (DAPI) from Santa Cruz Biotechnology Inc.
All data are presented as means ± standard deviation (SD). The mean values were calculated based on data from at least three independent experiments that were conducted on separate days using freshly prepared reagents. Data were analyzed using the analysis of variance (ANOVA) test of statistical package for the social sciences (SPSS Inc., Chicago, IL, USA). Significance differences between treatment groups were determined by using Duncan’s multiple range tests. The significance of differences was defined at p < 0.05.
>
Cytotoxicity of FcA and FcB in HT1080 cells
A significant part of the gelatinase pool is found in the media. Because serum contains gelatinases, it is necessary to prepare serum-free media for gelatin zymography. However, long incubation times in serum-free media can affect viability cell viability. To determine nontoxic concentrations of the fucoxanthin derivatives, the cytotoxic effects of FcA and FcB were examined using a MTT cell viability assay. No significant toxic effects were observed in the presence or absence of FBS treated with concentrations of these fucoxanthin derivatives up to 50 μM (Fig. 2). Thus, based on these results, MMP activity assays were examined up to the concentration of 50 μM.
>
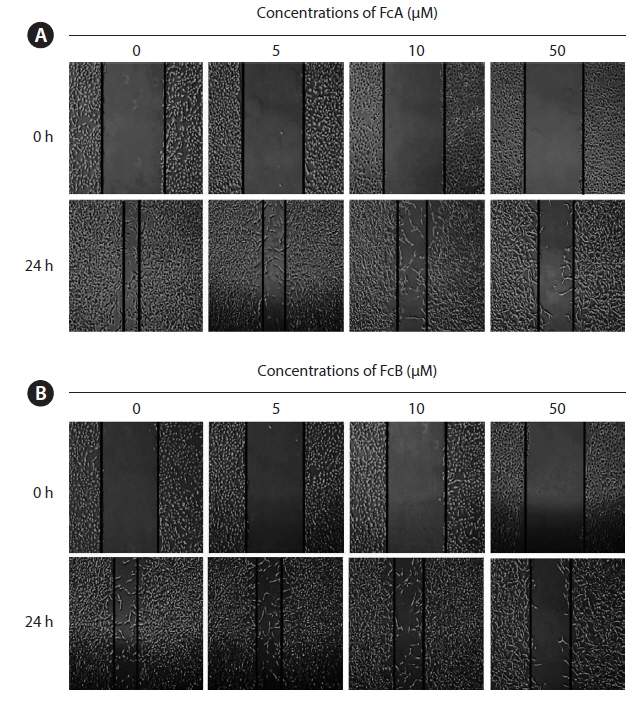
Effects of FcA and FcB on the migration of HT1080 cells
We next examined whether FcA and FcB inhibits HT1080 cell migration. The capacity for migration is a prerequisite for cell invasion through the basement membrane. The cells were treated with various concentrations of FcA and FcB (5, 10, and 50 μM). FcA and FcB suppressed the migration of HT1080 cells across the wounded space in a time and dose dependent manner (Fig. 3A & B).
>
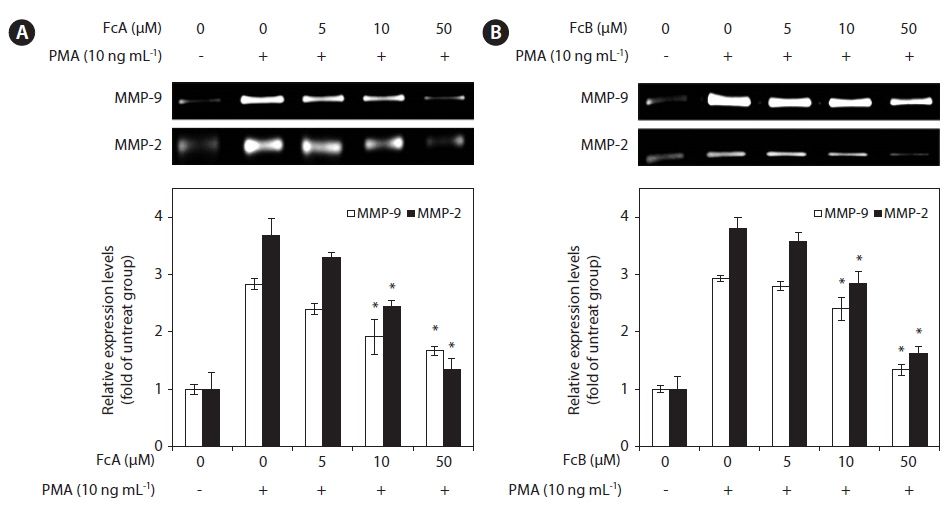
Inhibitory effect of fucoxanthin derivatives on MMP-2 and MMP-9 activities
In order to determine whether FcA and FcB affect the gelatinolytic activities of MMP-2 and MMP-9 secreted from HT1080 cells, the cells were pre-treated with various concentrations of FcA and FcB (5, 10, and 50 μM), MMP-2 and MMP-9 expression was stimulated using the potent tumor promoter, PMA. PMA enhanced the activity-based zymography of MMP-2 and MMP-9 when compared with untreated cells, whereas treatment with FcA and FcB significantly inhibited MMP-2 and MMP-9 activities (Fig. 4A & B).
>
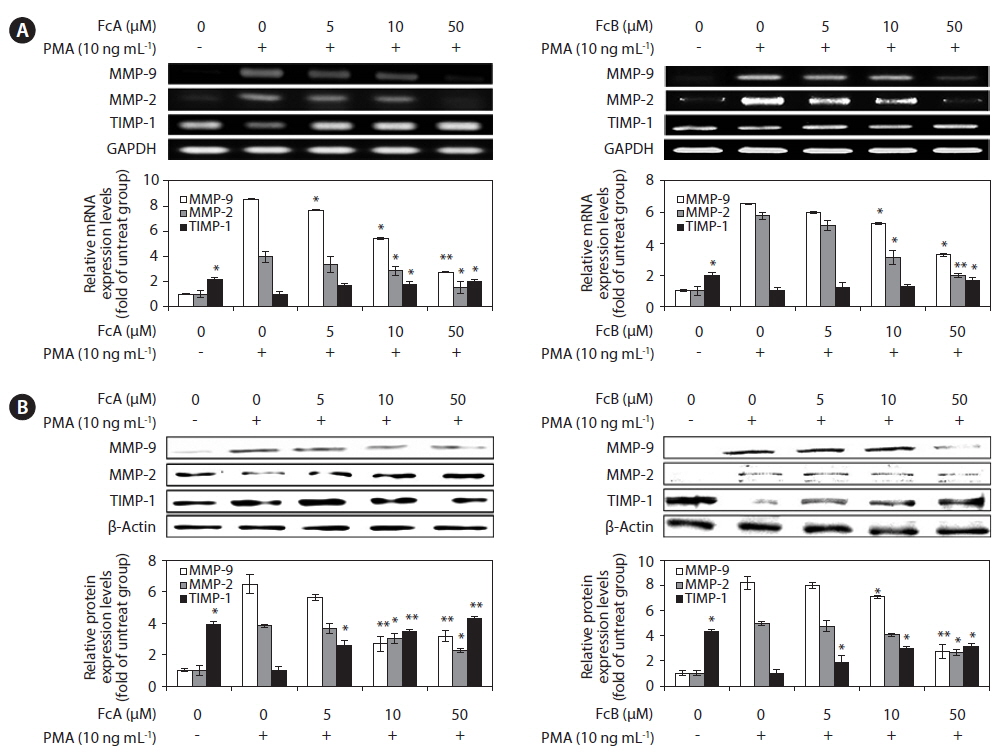
Effects of FcA and FcB on PMA-stimulated MMPs and TIMP-1 protein and mRNA expressions
We investigated whether FcA and FcB affects the expressed levels of MMP-2, MMP-9, and TIMP-1 protein and mRNA using Western blotting and RT-PCR (Fig. 5). As shown in Fig. 5A, treatment with FcA and FcB reduced the expression levels of MMP-2 and MMP-9 mRNA significantly when compared with the PMA stimulated group. TIMP-1 mRNA levels were increased by treatment in a dose dependent manner. Under the same condition, the levels of MMPs and TIMP-1 protein expression were correlated with their mRNA levels (Fig. 5B). Similar patterns were observed on PMA-stimulated MMPs and TIMP-1 protein and mRNA expression.
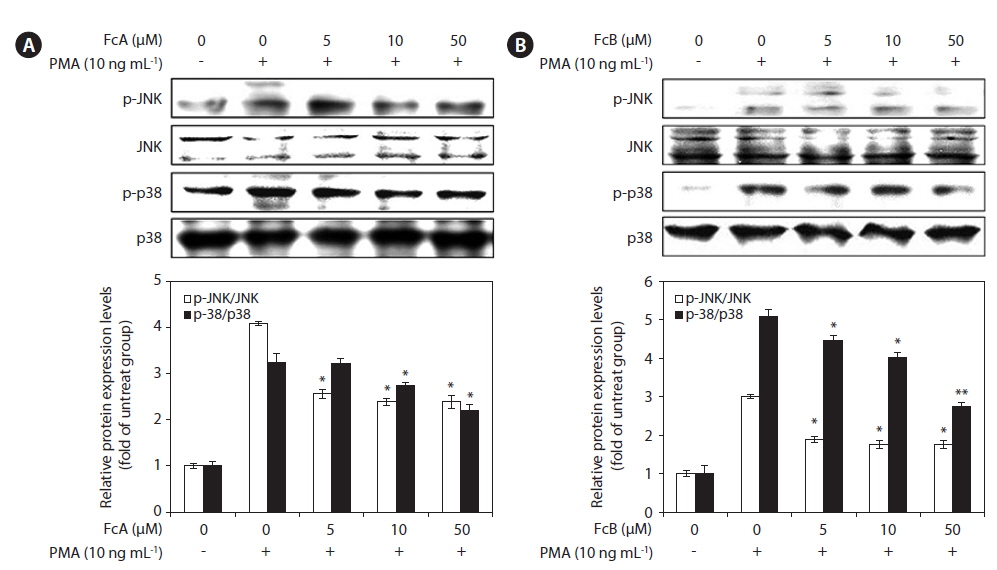
To determine whether FcA and FcB suppressed the activation of MMPs by on the MAPK pathway, we evaluated the effect of FcA and FcB on PMA-induced phosphorylation of p38 and JNK in HT1080 cells using Western blotting (Fig. 6). The stimulation of HT1080 cells with PMA resulted in increased phosphorylation of both types of MAPKs, JNK, and p38. However, when compared to the cells treated with just PMA, the HT1080 cells pre-treated with FcA and FcB exhibited significantly lower PMA-induced phosphorylation (Fig. 6A & B). This indicates that phosphorylation of MAPKs was effectively blocked by FcA and FcB in PMA-activated HT1080 cells.
>
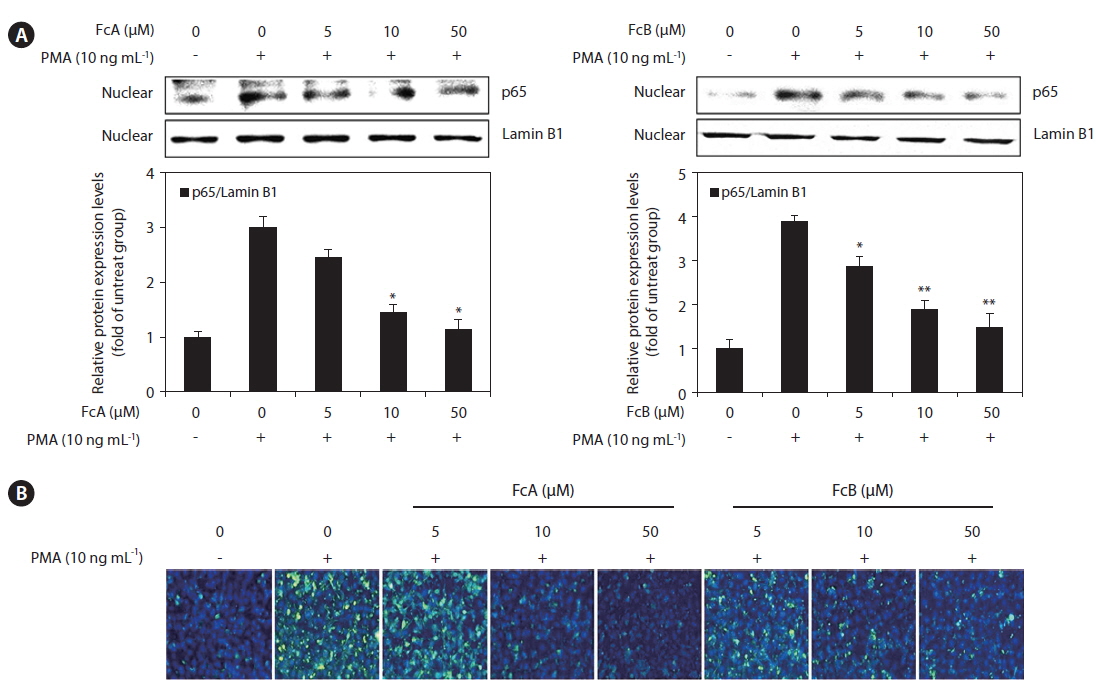
Effects of FcA and FcB on the activation of NF-κB in PMA-stimulated HT1080 cells
To elucidate the mechanism involved in the inhibition of MMPs and TIMPs by FcA and FcB in PMA-stimulated HT1080 cells, we studied the effect of FcA and FcB on NF-κB p65 translocation using a Western blot analysis. The translocation of NF-κB p65 was increased in response to PMA. FcA and FcB significantly suppressed NF-κB p65 translocation in a dose dependent manner (Fig. 7A). We also investigated the effects of FcA and FcB on NF-κB p65 expression levels using immunofluorescence (Fig. 7B). PMA treatment caused a significant increase in these levels, whereas treatment with FcA and FcB markedly reduced PMA-stimulated expression of NF-κB p65.
Marine algae are part of traditional remedies for healing and are believed to have profound curative properties. Previous reports found them to have various potential bioactivities. Some of active compounds were discovered to be chromenes, phlorotannins, and carotenoids (Jang et al. 2005, Kim et al. 2010). Dietary carotenoids have been implicated in biological processes, and havebeen demonstrated to have beneficial effects, including anti-inflammatory, anti-oxidant and anti-tumor activities (Shiratori et al. 2005, Heo et al. 2008, 2012, Kim et al. 2013). Among the carotenoids, fucoxanthin has an unusual allenic bond and 5,6-monoepoxide that contribute to its unique molecular structure (Yan et al. 1999, Heo et al. 2012). According to previous review, the allenic bond serves as a key structure for bioactivity of fucoxanthin, a marine nutraceutical from brown algae (Miyashita et al. 2011). Nakazawa et al. (2009) reported that
MMPs contribute significantly to the pathogenesis of inflammatory diseases, including atherosclerosis, neurodegenerative diseases, metastasis, and angiogenesis (Kong et al. 2008, Chou et al. 2010). Among the MMPs, MMP-2 can degrade type IV collagen, one of the major components of the basement membrane. This results in the promotion of tumor metastasis (Lee et al. 2007). MMP-9 is a pivotal member of the MMP family and is involved in the cleavage of all types of denatured collagens and of native basement membrane proteins (Mendis et al. 2006). The MMPs and TIMPs constitute an extracelluar proteolytic system whose coordination is critical for development, homeostasis, and injury repair (Chou et al. 2010). Also, both MMP-2 and MMP-9 are believed to play vital roles in tumor invasion and maigration (Kong et al. 2008). MMPs are secreted in some cancer cells and other various types of cells, and are potential targets of cancer therapy. Therefore, we tried to evaluate whether fucoxanthin derivatives might play a positive role in inhibiting MMPs. The fibroblasts play a central role in tissue remodeling, mostly in wound healing processes, and are involved in the pathogenesis of connective tissue diseases (Nguyen et al. 2013). In the present study, we selected human fibrosarcoma (HT1080) cells, since among the fibroblasts, the HT1080 cells have been used widely as model system to study MMP expression (Kong et al. 2008). The results of this study demonstrated that FcA and FcB significantly suppressed cancer cell migration and the activities of MMP-2 and MMP-9, both critical factors in tumor invasion and metastasis. The regulation of MMP activity by inhibiting active MMPs via naturally occurring endogenous TIMPs is important. Among the TIMPs, TIMP-1 is an endogenous inhibitor of MMPs (McCawley and Matrisian 1999). The down-regulation of MMP expression could be due to the up-regulation of its endogenous inhibitor, TIMP-1 (Rajapakse et al. 2006, Bauvois 2012). Interestingly, although treatment of HT1080 cells with PMA attenuated the expressed levels of TIMP-1, treatment with FcA and FcB significantly increased levels of TIMP-1 compared with the PMA-stimulated group.
To evaluate the mechanism of the inhibitory effect of FcA and FcB on MMPs activation, we examined the activation of transcription factors NF-κB and MAPKs. One of the most extensively investigated transduction mechanisms involved in the cell migration process is the MAPKs pathway (Kim et al. 2014). It was previously reported that MAPKs such as JNK and p38 play a role as upstream modulators in gelatinases gene expression (Mendis et al. 2009, Bauvois 2012, Park and Kim 2012). In addition, Hwang et al. (2010) and Kwak et al. (2006) reported that the PMA could effectively increase MAPK (JNK and p38) expression in HT1080 cells, and the down-regulation of MAPK may contribute to the inhibition of MMPs. Therefore, MAPK pathways are appropriate targets for the pharmacological treatment of tumor invasion and metastasis. Thus, we examined the inhibitory effect of FcA and FcB on the activation of MAPKs in PMA-stimulated HT1080 cells. FcA and FcB attenuated LPS-induced phosphorylation of p38 and JNK. The mechanism responsible for PMA-mediated responses in the cells treated with FcA and FcB may involve modulation of the transcription factor NF-кB. The NF-кB transcription family proteins consist of several protein subunits. Among them are the p65 subunits which contain transactivation domains necessary for gene induction. In addition, activation of NF-кB also requires the phosphorylation of MAPK signaling pathways (Kaomongkolgit et al. 2008, Hwang et al. 2010). Previous studies have reported that the NF-кB binding site was on the gene encoding MMPs, and NF-кB inhibitory signals can also inhibit the expression of MMP-2 and MMP-9 (Park et al. 2005, Nguyen et al. 2013, Zhao et al. 2013). Our study found that FcA and FcB inhibited PMA-stimulated NF-κB p65 activation. This suggests that the effects of FcA and FcB on the activation of MMPs are mediated, at least in part, by the suppression of the NF-κB signaling pathway.
In summary, we demonstrated that fucoxanthin derivatives (FcA and FcB) were potent inhibitors of cell migration and MMP activation in PMA-stimulated HT1080 cells. The levels of phosphorylated MAPKs in PMA-stimulated HT1080 cells were significantly decreased by treatment with FcA and FcB. Furthermore, these inhibitory effects were mediated through the inhibition of the translocation of NF-κB. These findings lead us to conclude that fucoxanthin derivatives appear to have the potential to prevent PMA-induced cell migration.




![Cytotoxic effects of fucoxanthin derivatives (9′-cis-(6′R) fucoxanthin [FcA] and 13-cis and 13′-cis-(6′R) fucoxanthin complex [FcB]) on HT1080 cells in the presence and absence of fetal bovine serum (FBS). Cells were treated for 24 h with various concentrations (5, 10, and 50 μM) of FcA (A) and FcB (B). Cytotoxicity was determined using the MTT assay. Values are expressed as mean ± standard deviation of triplicate experiments.](http://oak.go.kr/repository/journal/16474/JORHBK_2014_v29n4_355_f002.jpg)