미세아교세포는 신경교세포의 일종으로 중추신경계에서 식세포 역할을 담당하여 기본적인 면역방어체계의 역할을 수행하며, 중추신경계의 항상성 유지에 중요한 역할을 하는 것으로 알려져 있다1-3). 하지만 세균감염 후 lipopolysaccharide(LPS)와 같은 독소는 미세아교세포를 과다하게 활성화시켜 신경독소, 염증매개인자 및 염증성 사이토카인들의 분비가 증가하고, 결과적으로 신경퇴행성 질환의 원인이 되기도 한다4-6). 따라서 신경퇴행성 질환의 예방과 치료에 있어서 미세아교세포의 과도한 활성화의 억제는 신경퇴행성 질환 퇴치 후보 약물의 중요한 표적이 될 수 있을 것이다.
청뇌명신환(淸腦明神丸)은 동의대부속한방병원 신계내과 황원덕교수에 의해 작방된 것으로 기억력증진과 치매예방을 목적으로 개발된 환약이다. 본 처방은 원대(元代) 위역림(危亦林)이 황제께 진상한 공진단(拱辰丹)을 근간으로 하고 있다. 위역림의 의서인 세의득효방(世醫得效方)에 최초로 언급된 공진단에 대해 동의보감 잡병편 허로(東醫寶鑑 雜病篇虛勞)에서는 타고난 원기를 든든히 하여 오장자화(五藏自和), 백병불생(百病不生)하게 하는 데에 공진단을 쓴다고 하였다7). 본 환은 공진단의 사향(麝香), 녹용(鹿茸), 당귀(當歸) 등을 주재로 하였으며, 공진단의 산수유(山茱萸)를 산약(山藥)으로 바꾸었고, 본방에 용안육(龍眼肉), 원지(遠志), 인삼(人蔘), 육계(肉桂), 부자(附子), 침향(沈香), 용뇌(龍腦)를 추가하여 구성되어져 있다. 본 연구에 사용된 청뇌명신환은 황원덕교수의 위탁을 받아 저자가 임상에서 사용중인 처방이다.
그러나 청뇌명신환의 효능에 비하여 그에 대한 실험적인 근거는 전무한 실정이다. 따라서 본 연구에서는 LPS에 의해 자극된 BV2 생쥐 미세아교세포의 염증반응 억제에 미치는 청뇌명신환의 기전을 조사하고, 신경퇴행성 질환 예방 및 치료 후보제로서의 청뇌명신환의 효능을 확인하였다.
본 실험에 사용된 청뇌명신환(淸腦明神丸, Cheongnoimyungshin-hwan, CNMSH)은 인삼(Ginseng Radix Alba), 당귀(Angelicae Gigantis Radix), 산약(Dioscoreae Rhizoma)과 용안육(Longan Arillus)을 각 24 g, 육계(Cinnamomi Cortex) 12 g, 원지(Polygalae Radix) 6 g, 사향(Moschus)과 침향(Aquilariae Lignum) 각 1 g, 용뇌(Borneolum, 중국) 0.2 g 이상을 세말한 후, 여기에 녹용(cornus cervi parvum) 24 g과 부자(Aconiti Lateralis Radix Preparata) 6 g을 5시간 정도 끊인 교(膠) 24 g을 추가한 후, 꿀 160 g으로 구성된 총 300.2 g의 환약을 4 g 씩으로 75개를 만든 후, 무작위 1환을 선정하여 금박으로 입혀서, 이를 멸균된 3차 증류수를 이용하여 100 mg/ml의 농도로 만든 다음 0.2 μm 필터로 여과 후 적정 농도로 배지에 희석하여 처리하였다.
본 실험에서 단백질 분석을 위하여 사용된 항-IL-1β, 항-β-actin, 항-extracellular signal-regulated kinase (ERK), 항-p38 mitogen-activated protein kinase (MAPK) 및 항-Akt 항체는 Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA)에서 구입하였으며, TNF-α, pERK, c-Jun amino terminal kinase (JNK), p-JNK, p-p38 MAPK, nuclear factor κB (NF-κB) (p65)-s468, NF-κB (p65)-s538 항체는 Cell Signaling Technology, Inc. (Danvers, MA, USA)에서 구입하였다. Immunoblotting을 위해 2차 항체로 사용된 horseradish peroxidase (HRP)-conjugated antimouse 및 anti-rabbit 항체는 Santa Cruz Biotechnology Inc.에서 구입하였다.
실험에 사용된 생쥐 신경소교세포주인 BV2 세포는 인제대학교 의과대학 미생물학교실에서 분양 받아 사용하였으며, 세포의 배양을 위해 10%의 우태아혈청 (fetal bovine serum, FBS, Gibco BRL, Grand Island, NY, USA)과 1%의 penicillin과 streptomycin이 포함된 Dulbecco’s Modified Eagle’s Medium (DMEM) 배지(Gibco BRL)를 사용하여 37℃, 5% CO2 조건 하에서 배양하였다. 세포수의 증식에 따른 과밀도 현상을 해소하기 위하여 매 48시간마다 계대배양을 실시하여 적정수의 세포를 유지하였다.
청뇌명신환 처리에 의한 세포의 성장억제 정도를 확인하기 위하여 tetrazolium bromide salt (MTT) assay를 이용하였다. 먼저 6 well에 배양된 BV2 세포에 청뇌명신환 (0~500 ug/ml)과 LPS (500 ng/ml) (Sigma-Aldrich Chemical Co., St. Louis, MO, USA)를 상기 농도로 처리하고 24시간 동안 배양한 후 배지를 제거하고 MTT (Sigma-Aldrich Chemical Co.)를 0.5 mg/ml 농도로 희석하여 200 μl씩 분주하고 37℃에서 3시간 동안 다시 배양하였다. 배양이 끝난 다음 MTT 시약을 제거하고 dimethylsulfoxide (DMSO, Sigma-Aldrich Chemical Co.)를 1 ml씩 분주하여 well에 생성된 formazin을 모두 녹인 후 96 well plate에 200 μl씩 옮겨서 ELISA reader (Molecular Devices, Sunnyvale, CA, USA)로 540 nm에서 흡광도를 측정하였다. 측정은 모두 세번을 하였으며, 그에 대한 평균값과 표준 오차를 Microsoft EXCEL program을 사용하여 분석하였다.
NO의 농도는 배양액 내의 nitrite농도를 Griess Reagent (Sigma-Aldrich Chemical Co.)를 이용하여 측정하였다. 이를 위하여 BV2 세포에 각 농도별로 청뇌명신환을 1시간 선 처리한 후 500 ng/ml의 LPS를 처리하여 24시간 배양한 후 세포 배양액을 수거하였다. 배양액 100 μl와 동량의 Griess Reagent를 상온에서 반응 시킨 후 ELISA reader로 540 nm에서 흡광도를 측정하였는데, sodium nitrite (NaNO2)의 농도 별 표준곡선을 이용하여 배양액 내의 NO 농도를 결정하였다.
PGE2 생성량 측정을 위해 사용한 PGE2 enzyme immunoassay (EIA) kit는 Cayman Chemicals Co. (Ann Arbor, MI, USA)에서 구입하였다. BV2 세포에 125, 250, 500 ug/ml 농도의 청뇌명신환을 1시간 선 처리 후, LPS (500 ng/ml)를 처리한 배지에서 24시간 동안 세포를 배양시킨 후 상층액만 이용하여 PGE2 EIA kit에 제시된 방법에 따라 처리한 다음 ELISA reader를 이용한 420 nm의 흡광도로 반응의 정도를 비교하였다.
세포배양액 내의 pro-inflammatory cytokine (IL-1β 및 TNF-α)의 양을 측정하기 위한 Quantikine enzyme-linked immunospecific assay (ELISA)는 R&D systems (Minneapolis, MN, USA)에서 구입하였다. 동일한 조건에서 배양된 BV2 세포의 배양액을 이용하여 IL-1β 및 TNF-α의 생성양을 제시된 방법에 따라 처리한 다음 ELISA reader를 이용한 450 nm의 흡광도로 반응의 정도를 측정하였다.
8. Western blot analysis에 의한 단백질 발현의 분석
준비된 세포에 적당량의 lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM dithiothreitol (DTT)]를 첨가하여 4℃에서 1시간 동안 반응시킨 후, 14,000 rpm으로 30분간 원심 분리하여 상층액에 있는 총 단백질을 분리하였다. 상층액의 단백질 농도는 Bio-Rad 단백질 정량 시약(Bio-Rad, Hercules, CA, USA)과 그 사용방법에 따라 정량한 다음 동량의 Laemmli sample buffer (Bio-Rad)를 섞어서 sample을 만들었다. 동량의 sample을 sodium dodecyl sulphate (SDS)-polyacrylamide gel을 이용하여 전기영동으로 분리한 후, nitrocellulose membrane (Schleicher and Schuell, Keene, NH, USA)으로 electroblotting에 의해 전이시켰다. 분리된 단백질이 전이된 nitrocellulose membrane을 5% skim milk를 처리하여 비특이적인 단백질들에 대한 blocking을 실시하고 1차 항체(Table 1)를 처리하여 상온에서 2시간 이상 또는 4℃에서 over night 시킨 다음 PBS-T로 세척(15분간 1번, 5분간 5번)하고 처리된 1차 항체에 맞는 2차 항체(PBS-T로 1:1,500으로 희석하여 사용)를 사용하여 상온에서 1시간 정도 반응시켰다. 반응이 끝난 후 암실에서 Enhanced Chemiluminoesence (ECL) solution (Santa Cruz Biotechnology Inc.)을 적용시킨 다음 X-ray film에 감광시켜 특정단백질의 발현양을 분석하였다.
[Table 1.] Antibodies Used in the Present Study
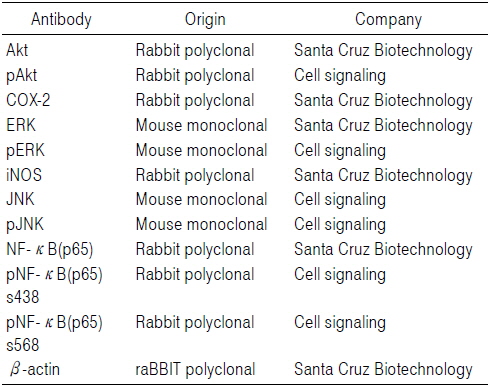
Antibodies Used in the Present Study
다양한 실험으로부터 얻은 결과는 mean±standard error로 기록하였고, 유의성 검증은 one-way ANOVA 분석법을 이용하여 결정하였다.
1. BV2 생쥐 미세아교세포 생존도에 미치는 청뇌명신환 영향
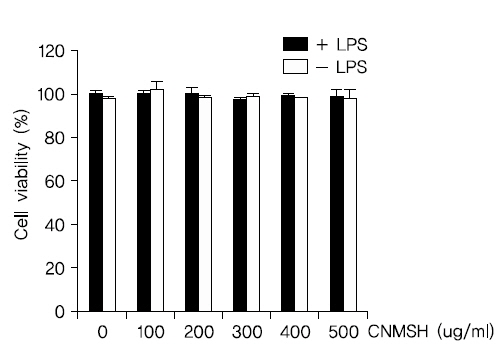
가장 먼저 생쥐 미세아교세포 BV2에서 항염증 효과에 대한 청뇌명신환의 농도 범위를 설정하기 위해 MTT 분석을 이용하여 세포 생존도를 측정해 보았다. Fig. 1에서 보이는 바와 같이, 청뇌명신환을 0~500 ug/ml 범위 내에서 LPS 500 ng/ml과 복합 및 단독처리 하였을 때, BV2 생쥐 미세아교세포의 세포 생존도에는 영향을 미치지는 않는다는 것을 확인할 수 있었다. 이에 향후 실험에서는 청뇌명신환의 최고 처리농도를 500 ug/ml으로 설정하여 진행하였다.
2. NO 생성과 inducible NO synthese (iNOS)의 발현에 미치는 청뇌명신환 영향
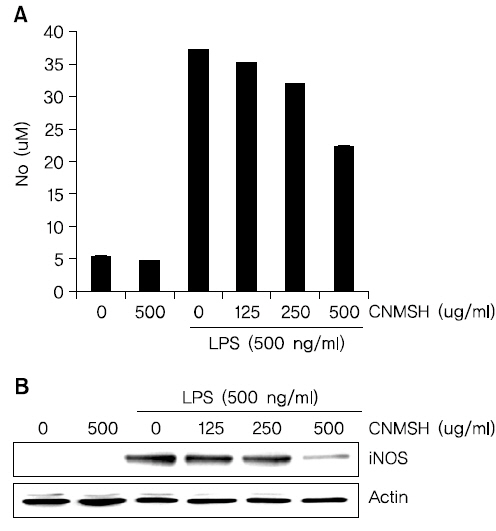
Fig. 1의 결과를 바탕으로 청뇌명신환의 처리 농도(125~500 ug/ml)를 설정 이후에 BV2 생쥐 미세아교세포에서 LPS에 의해 유도되는 NO의 생성과 iNOS 단백질 발현의 증가에 미치는 영향에 대해 조사하였다. Fig. 2A에 나타낸 바와 같이 LPS 500 ng/ml의 단독 처리에 의해 현저히 증가된 NO의 생성이 각 청뇌명신환의 선 처리에 의해 현저히 억제 되는 것이 확인되었다. NO 뿐만 아니라 NO를 합성하는데 영향을 미치는 iNOS 단백질을 Western blotting을 통해 확인해 본 결과, NO 생성 억제와 유사하게 iNOS 단백질의 발현이 청뇌명신환 처리 농도 의존적으로 억제되었음을 알 수 있었다(Fig. 2B). 이상의 결과에서 염증 반응에 중요한 매개체로 작용하는 NO의 생성을 청뇌명신환이 iNOS의 발현을 억제함으로서 효과적으로 차단할 수 있다는 것이 확인되었다.
3. PGE2의 생성과 cyclooxygenase-2 (COX-2)의 발현에 미치는 청뇌명신환 영향
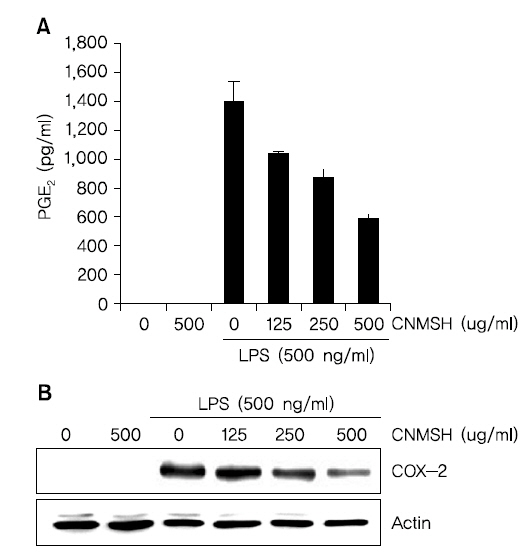
BV2 생쥐 미세아교세포에 최대한 독성이 없는 농도 범위에서 LPS에 의해 유도되는 PGE2의 생성과 COX-2 단백질의 발현에 미치는 영향에 대해 조사하였다. Fig. 3A에서 나타낸 바와 같이 LPS 500 ng/ml 처리에 의해 현저히 증가된 PGE2의 생성이 청뇌명신환의 선 처리에 의해 처리 농도 의존적으로 현저히 억제 되는 것이 확인되었다. 뿐만 아니라 PGE2를 합성하는데 영향을 미치는 COX-2 단백질을 Western blotting을 통해 확인해 본 결과, PGE2 생성 억제와 유사하게 청뇌명신환 선처리에 의해서 LPS에 의해 증가된 COX-2 단백질의 발현이 억제되었음을 알 수 있었다(Fig. 3B). 이상의 결과에서 염증 반응에서 발열 및 통증에 매개체로 작용하는 PGE2 생성을 청뇌명신환이 COX-2의 발현을 억제함으로써 효과적으로 차단할 수 있음을 알 수 있었다.
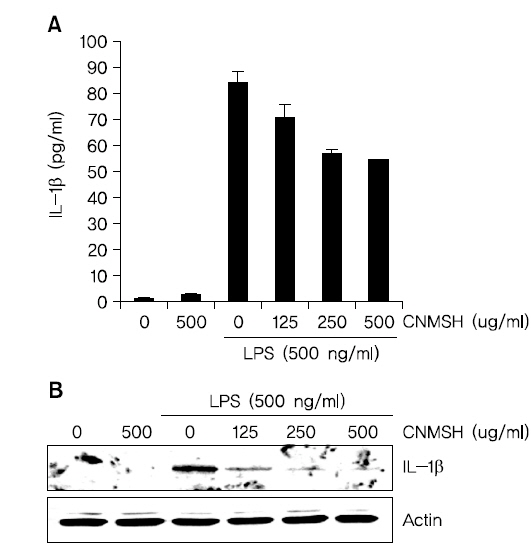
4. IL-1β 생성과 단백질 발현에 미치는 청뇌명신환 영향
IL-1β는 염증성 cytokine으로 활성화된 미세아교세포에서 생산되어 염증 반응의 중요한 중개자 역할 뿐만 아니라 세포 증식, 분화 및 세포자살 등의 여러 가지 세포 활동에 다양하게 관여하고 있다8). 그에 따라 LPS에 의해 활성화된 BV2 미세아교세포에 유도된 IL-1β 생성 및 단백질 발현 증가에 대한 청뇌명신환의 영향을 조사해 보았다. Fig. 4에 나타낸 바와 같이 LPS 500 ng/ml 처리에 의해 현저히 증가된 IL-1β 생성과 단백질의 발현이 청뇌명신환의 선 처리에 의해 처리 농도 의존적으로 현저히 억제되었음을 확인하였다. 이상의 결과에 따라 IL-1β의 생성과 단백질 발현을 청뇌명신환이 효과적으로 억제할 수 있다는 것을 알 수 있었다.
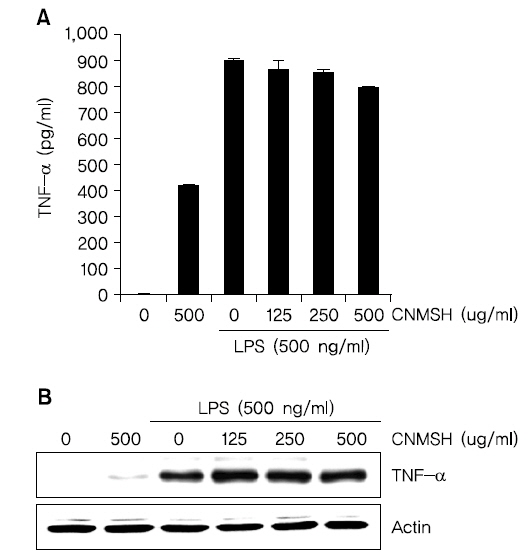
5. TNF-α 생성과 단백질 발현에 미치는 청뇌명신환 영향
TNF-α는 가장 대표적인 cytokine으로 면역세포를 조절하는 역할을 한다. 하지만 TNF-α 생산 조절 장애는 치매, 암을 포함한 다양한 인간 질병에 관여한다고 알려져 있다9,10). 그에 따라 LPS에 의해 활성화된 BV2 생쥐미세아교세포에 유도된 TNF-α의 생성 및 단백질에 대한 청뇌명신환의 영향을 조사해 보았다. Fig. 5에 나타낸 바와 같이 청뇌명신환 500 ug/ml의 단독처리군에서도 TNF-α 생성이 어느 정도 증가되었을 뿐 아니라, LPS 처리에 의해서 증가된 TNF-α 생성과 발현 억제에도 청뇌명신환은 크게 영향을 미치지 못하는 것으로 확인 되었다. 이상의 결과에 따라 TNF-α의 생성과 단백질 발현에는 청뇌명신환이 유의적인 효과가 없음을 알 수 있었다.
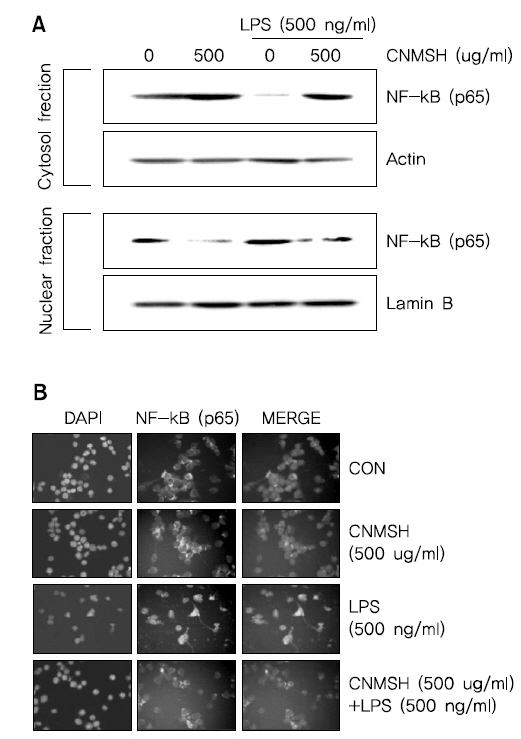
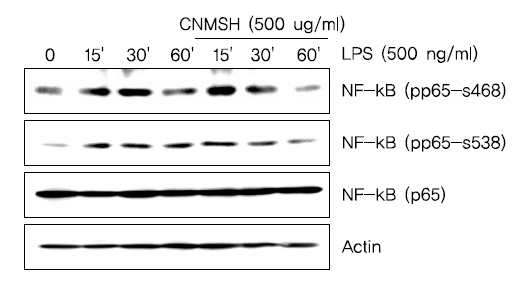
6. NF-κB의 활성화 및 인산화에 미치는 청뇌명신환의 영향
활성화된 미세아교세포에서 NF-κB는 앞서 확인한 iNOS, COX-2, IL-1β 및 TNF-α 등의 발현을 조절하는 주요 전사조절인자로서 작용한다고 알려져 있다11-13). 이에 따라 LPS에 의한 염증반응에서 NF-κB 활성 증가에 미치는 인산화 억제를 통해 이루어진다는 것을 확인할 수 있었다.다. 먼저 핵과 세포질의 단백질을 분리하여 NF-κB의 subunit의 하나인 p65 단백질의 발현변화를 확인해 본 결과, LPS 처리에 의해 핵 내로 축적된 p65 단백질이 청뇌명신환 선처리에 의해서 감소되는 것이 확인되었다(Fig. 6A). 뿐만 아니라 면역형광염색법을 이용하여 p65 단백질의 세포 내 위치 변화를 확인 본 결과, LPS 처리에 의해 핵 내로 이동한 p65 단백질이 청뇌명신환 선처리에 의해서 이동이 억제되었음을 알 수 있었다(Fig. 6B). 그리고 NF-κB의 활성화는 인산화 과정을 동반한다고 알려져 NF-κB p65 단백질의 인산화 변화를 조사해 본 결과, LPS 처리 15분부터 증가되었던 NF-κB p65의 인산화가 청뇌명신환 선처리에 의해서 현저히 억제되었음을 알 수 있었다(Fig. 7). 이상 결과에서 청뇌명신환의 의한 NF-κB의 핵내로의 이동 및 활성억제는 NF-κB p65 인산화 억제를 통해 이루어진다는 것을 확인할 수 있었다.
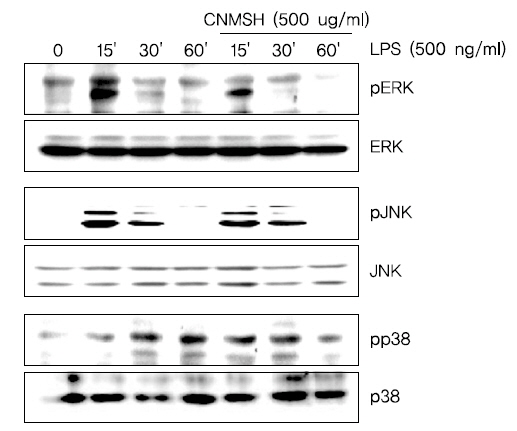
7. MAPK 신호전달계의 활성화에 미치는 청뇌명신환의 영향
다음은 세포신호전달계 중 가장 대표적인 MAPK 신호전달계와 청뇌명신환의 항염증효과와의 상관관계를 조사하였다. MAPK 신호전달계는 일반적으로 ERK, JNK 및 p38 MAPK 세 가지 kinase로 이루어져 있으며, 이 단백질들은 하위에 여러가지 표적 단백질을 인산화시켜 활성화를 조절하는 것으로 보고되어 지고 있다14-16). Fig. 8에서 보이는 바와 같이, ERK, JNK 및 p38 MAPK 모두 LPS 처리 15분 이내에 인산화형이 증가되어 활성화되었음을 알 수 있었으며, JNK를 제외한 ERK와 p38 MAPK는 청뇌명신환 선처리에 의하여 LPS에 의한 활성이 억제되었음을 확인하였다. 이상의 결과에 따라 청뇌명신환의 항염증 효과에 MAPK 신호전달계 중 ERK와 p38 MAPK가 관여하고 있음을 확인할 수 있었다.
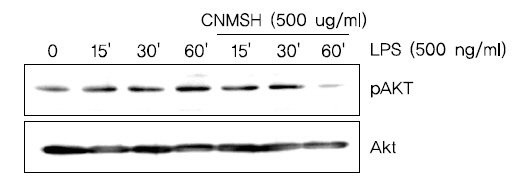
8. Phosphoinositide 3-kinase (PI3K)/Akt 신호전달계의 활성화에 미치는 청뇌명신환의 영향
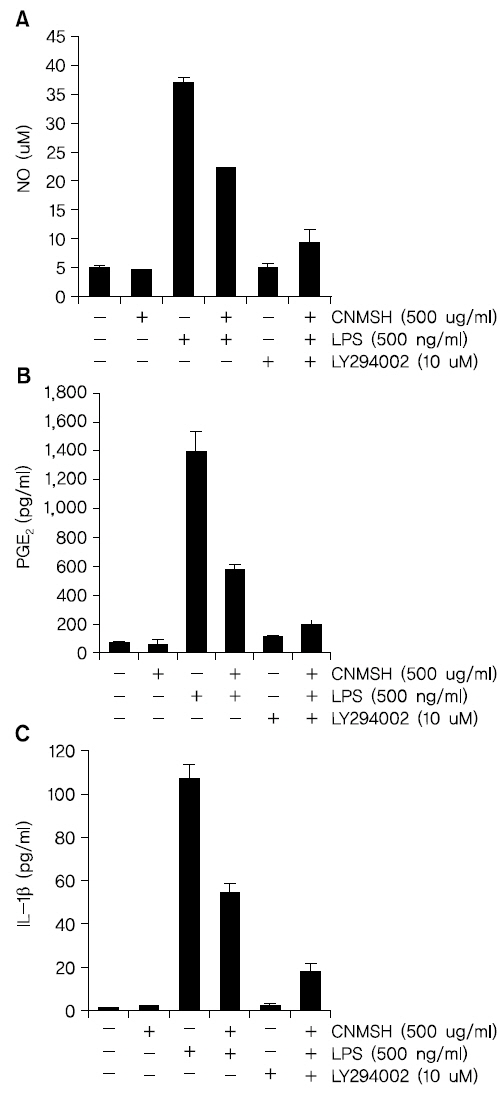
PI3K/Akt 신호전달계는 NF-κB의 활성을 조절할 수 있는 상위 세포 신호전달계의 중 하나이다17,18). 이에 PI3K/Akt 신호전달계와 청뇌명신환의 항염증효과와의 상관관계를 조사하였다. Fig. 9에서 보이는 바와 같이, LPS 처리에 의해 15분 이내에 활성화되었던 PI3K의 하위 신호 단백질인 AKT가 청뇌명신환 선 처리에 의하여 활성이 억제되는 것이 확인되었다. 뿐만 아니라 PI3K의 억제제로 알려져 있는 LY294002를 청뇌명신환과 동시에 처리할 경우, 청뇌명신환 단독 처리군에 비하여 항염증 효과가 증가되는 것을 확인할 수 있었다(Fig. 10). 이상의 결과에 따라 청뇌명신환의 항염증 효과에 PI3K/Akt 신호전달계 역시 중요한 상위 신호계로 작용하고 있음을 알 수 있었다.
과다 활성화된 미세아교세포에 의해 야기되는 뇌 염증은 치매, 파킨슨병, 다발성 경화증 및 근위축성 축삭 경화증을 포함한 여러 신경퇴행성 질환의 병리학적 특징이다19-21). 따라서 과다 활성화된 미세아교세포의 조절은 많은 신경변성 질환의 치료를 위한 유망한 치료법이 될 수 있을 것이다.
본 연구에서는 청뇌명신환의 뇌세포 보호 효과 및 관련 기전의 확인을 위한 선행 연구로서, LPS에 의해 활성화된 BV2 미세아교세포에서 염증 매개 인자와 염증성 사이토카인의 생성에 미치는 청뇌명신환의 영향을 조사하였다.
청뇌명신환은 원대 위역림이 황제께 진상한 공진단을 근간으로 하여, 공진단의 산수유를 산약으로 바꾸었고, 본방에 인삼, 육계, 부자, 용안육, 원지, 침향, 용뇌를 추가하여 구성된 처방이다. 이상의 약재들을 살펴보면, 현대인들의 위장운동의 부담을 덜어 주기 위하여 공진단의 산수유를 산약으로 바꾸었고, 인삼, 육계, 부자는 전신 기능 활성화를 목적으로 추가하였으며, 용안육, 원지, 침향, 용뇌 등은 뇌의 기능을 활성화하여 현대인의 정신적인 스트레스로 생긴 기억력감퇴 및 치매예방을 목적으로 추가하였다. 산수유의 성미(性味)는 산(酸), 미온(微溫)하며, 정기(正氣), 진액(津液)을 수렴하는 의미가 강하다22). 산약의 성미(性味)는 온무독(溫無毒), 고감(苦甘)하여 비신경(脾腎經)에 귀경(歸經)하여 보비익위(補脾益胃), 조습화중(燥濕和中)하는 효능이 있다. 또한 위장관 기능의 문제로 인한 질병 치료에 효능이 있음이 전23), Nishimura N24) 등의 연구에서 밝혀졌다. 그러므로 위장운동의 부담을 덜어주기 위한 목적으로 산수유를 산약으로 대체하여 본 처방에서 사용되었다. 인삼은 최근 허25), 김26), 진27) 등의 연구에 의하여 신경세포 손상 방어효과가 있음이 보고되었다. 육계는 고28)의 신생혈관 촉진 생성 작용, 김29)의 대식세포의 염증반응에 미치는 영향 등이 보고되고 있다. 부자는 Zhu Z30)의 비위온보(脾胃溫補) 기능에 관한 연구가 보고되었다. 따라서 인삼, 육계, 부자는 여러 기전을 통해 전신 기능 활성화를 목적으로 사용되었다. 용안육은 대식세포의 염증반응과 cytokine에 영향을 미쳐 항염증효과가 있음이 김31)의 연구에서 보고되었다. 원지는 최신 연구에서 TNF-α와 IL-1β 등의 염증성 사이토카인을 억제하고 NF-κB pathway에 작용하는 기전이 밝혀졌다32). 침향은 비(脾), 폐(肺), 신(腎)의 기체(氣滯)로 인한 증상을 소통하여 치료하는 약재로서33), 최근 Kim YC44)의 과민반응 억제에 관한 연구가 보고된 바 있다. 용뇌는 항 뇌빈혈 효능이 있음이 Kong QX34)의 연구에서 보고되었다. 이상의 연구 및 문헌에 근거하여 용안육, 원지, 침향, 용뇌를 뇌의 기능을 활성화하여 현대인의 정신적인 스트레스로 생긴 기억력감퇴 및 치매예방을 목적으로 사용하였다.
몇몇 연구에서는, 과다 유도된 COX-2의 활성 억제가 허혈성 뇌손상을 줄이고 치매와 파킨슨병의 진행을 느리게 할 수 있다고 보고되어지고 있다35,36). 또한 활성화된 미세아교세포에서 iNOS 발현 증가에 따른 NO의 과도한 생성 및 세포 외로의 방출은 신경퇴행성질환의 유발에 중요한 매개인자 및 촉진자로 작용한다5,37,38). 본 연구에서는 청뇌명신환의 선 처리에 의해 COX-2와 iNOS의 단백질 발현을 현저히 억제하였을 뿐 만 아니라, PGE2와 NO 생성 역시 청뇌명신환의 처리 농도 의존적으로 현저히 감소되었다(Fig. 2, 3). 그리고 이러한 항염증 효과가 나타난 농도 범위에서 청뇌명신환의 세포독성은 나타내지 않았다(Fig. 1). 활성화된 미세아교세포는 신경 염증을 유발하고, 이때 TNF-α와 IL-1β와 같은 염증성 사이토카인을 과도하게 생산 분비한다39,40). 이러한 염증성 사이토카인의 과도한 분비는 신경세포의 손상을 유도하는 것으로 밝혀져 있다. 따라서 이러한 염증성 사이토카인의 과도한 생산을 억제하는 것 또한 퇴행성신경질환의 예방을 위해 매우 중요하다41,42). 본 연구결과에서 청뇌명신환의 선 처리는 LPS에 의해 활성화된 BV2 미세아교세포에서 증가된 IL-1β의 단백질 발현과 분비를 억제하였으나(Fig. 4), TNF-α의 발현에는 그다지 영향을 주지 못하는 것을 확인하였다(Fig. 5). 비록 TNF-α의 발현과 생성에 대한 추가적인 연구가 필요하지만, 청뇌명신환은 최소한 IL-1β의 생성을 억제함으로서 항염증 효능을 지니고 있음을 알 수 있었다.
이상 활성화된 미세아교세포의 염증성 매개인자와 사이토카인의 과잉생산은 많은 세포신호전달경로를 통해 신경퇴행성 질환을 유발하는 위험 요인이 될 수 있다. 그 중에서도, 전사조절인자인 NF-κB는 염증성 유전자의 전사를 유도하는 특정 promoter 영역에 NF-κB가 결합하여 염증성 유전자의 전사를 유도하는 중요한 염증반응의 조절자이다43,44). 또한 NF-κB의 활성화를 통한 미세아교세포의 염증관련 유전자들의 발현은 PI3K/AKT경로가 관련한다는 것이 보고되어지고 있다45-47). 다양한 선행 연구 결과에 의하면 미세아교세포에서 과도한 NF-κB의 전사활성 및 PI3K/AKT 신호전달경로의 차단은 iNOS, COX-2 및 IL-1β 같은 염증성 사이토카인과 매개인자의 발현을 억제할 수 있다고 보고되어지고 있다48,49). 따라서 NF-κB와 PI3K/AKT 경로를 조절하는 것은 많은 신경퇴행성 질환 치료를 위한 유망한 신호전달경로로 인식되어지고 있다. 본 연구에서는 LPS에 의해 활성화된 BV2 미세아교세포에서 청뇌명신환은 NF-kB p65 단백질의 핵 내 이동을 억제하였을 뿐 아니라 NF-kB가 활성화되었음을 의미하는 인산화 역시 억제시켰다(Fig. 6, 7). 또한 청뇌명신환은 LPS에 의해 자극된 BV2 미세아교세포에서 AKT의 활성화를 억제하는 것으로 나타났으며, 이는 PI3K/AKT 신호전달경로의 불활성화에 의해 LPS에 의한 NF-κB 활성화와 염증매개인자들의 증가가 청뇌명신환에 의해 억제될 수 있을 것으로 사료되는 결과이다(Fig. 9, 10). 따라서 NF-κB와 PI3K/AKT 신호 전달 경로 억제는 청뇌명신환에 의한 미세아교세포의 항염증 효과에서 중요한 조절자로서의 역할을 수행할 것으로 예상된다.
MAPKs는 ERK, JNK 및 p38 MAPK를 포함하는 신호전달경로로 면역 및 염증성 시스템의 중요한 신호 전달 반응을 매개하고, 세포 분열, 세포 증식 및 유전자 발현을 포함하여 세포 활동의 중요한 조절자로서의 역할을 수행한다50-52). 일반적으로 JNK 및 p38 MAPK는 IL-1β, TNF-α, 세균내독소(LPS) 또는 세포 스트레스와 같은 염증성 사이토카인에 의해 활성화되고, ERK는 세포 분열 촉진과 같은 자극에 의해 활성화된다53). 따라서 본 연구에서 LPS에 의하여 활성화된 BV2 미세아교세포에서 청뇌명신환의 항염증 효과를 MAPK 활성과의 관계를 추가적으로 조사하였다. 본 실험의 결과는 LPS의해 ERK, JNK 및 p38 MAPK 등 MAPKs가 모두 인산화가 증가되었지만, 청뇌명신환의 선처리에 의해서 ERK 및 p38 MAPK의 인산화가 억제되었으나 JNK에는 큰 영향을 미치지 못하였다(Fig. 8). 따라서 MAPK 신호 전달 경로 역시도 청뇌명신환에 의한 미세아교세포의 항염증효과에 관련한다는 것 확인할 수 있었다.
본 연구의 결과를 요약해보면, 청뇌명신환이 PI3K/Akt신호전달계의 활성억제를 통하여 NF-κB의 활성을 차단함으로써 염증 매개 유전자인 COX-2, iNOS 및 염증성사이토카인 IL-1β와 그 산물인 PGE2, NO, IL-1β의 생성을 억제하였으며, 이는 청뇌명신환이 강력한 항염증 효능을 가지고 있음을 의미한다. 따라서 청뇌명신환은 미세아교세포에 대한 항염증효과는 퇴행성 뇌질환의 중요한 원인이 되는 만성적인 신경계 염증을 억제함으로써 신경퇴행성 질환 등 다양한 신경계 질환에서 신경보호역할을 할 것으로 기대된다.