



The olive fruit fly
Currently, there are several control options for reducing populations of olive fruit fly including neurotoxic insecticides, insect hormones, bait sprays (Kakani & Mathiopoulos, 2008; Skouras
Additionally, other factors including general deterioration of strain quality due to colonization for many generations (bottleneck effects), excessive homozygosis or other divergences from the original population may affect the maintenance of healthy strains under mass-rearing conditions (Joslyn, 1984). Therefore, several basic protocols for the proper maintenance of populations under mass-rearing conditions have been developed, including re-establishing the lineage through relaxed-rearing, artificial selection of more resistant lineages, hybridization of compatible lineages, and periodically supplying (refreshing) the colony with wild individuals (Leppla
To successfully establish a new colony of olive fly requires the adaptation of a wild population to artificial laboratory conditions. A major problem for the development of an efficient mass-rearing system for olive fly has been the reluctance or refusal of wild female flies to readily oviposit fertile eggs in any artificial medium; only olive fruit has so far been used for initial egg collections. Due to the relatively high cost of olive fruits compared with other more simple materials and inconsistent olive fruit supply year round, establishment of wild colonies of olive fly could be much facilitated if alternative methods of egg collection can be developed.
The current study initially focused on assessing the possibility of hybridizing wild male olive flies with laboratory adopted female olive flies to enhance egg production and facilitate the establishment of refreshed stable olive fly colonies under artificial rearing conditions. Second, the paper presents data regarding the use of table grapes as an alternative egg collection medium for four wild populations of olive flies. Finally, production characteristics of the four newly established olive fly colonies for the initial five generations are also presented.
Olive flies were maintained at the Insect Pest Control Laboratory (IPCL) of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Seibersdorf, Austria. For the hybridization experiment, wild olive fly pupae and infested olive fruits were collected from France (Neziganl'ereque area) in 2010, and they were crossed with laboratory adapted flies that were derived from a colony that had been cultured in Greece (Democritus strain) for approximately 300 generations (>30years) and that was introduced in the IPCL in 2002 (~120 generations at IPCL by the time of experiments).
In 2011, a second collection of infested olive fruits from the same location in France (termed France-Wild), and additionally Spain (Valencia; termed Spain-Wild), Italy (Ospedaletti; termed Italy-Wild) and Croatia (Dalmatia; termed Croatia-Wild) took place. The infested fruits were kept in plastic trays covered with a nylon bag to avoid contamination with
An artificial larval diet (Tsitsipis, 1975) was used in the study. The diet consisted of tap water (550 mL), extra virgin olive oil (20 mL), Tween 80 (7.5mL), potassium sorbate (0.5 g), nipagin (2 g), sugar (20 g), brewer's yeast (75 g), soy hydrolysate (30 g), hydrochloric acid 4.5ml, and cellulose powder (275 g). The eggs were seeded on the diet in fiberglass trays (38 cm × 7 cm × 4.5 cm) that were wrapped with thin plastic wrap and covered with another tray. The plastic wrap on each tray was removed after five days and trays were placed in larger trays with sawdust for pupae collection. Two to three days before onset of adult emergence, pupae were counted and placed in big Plexiglas cages for adult emergence.
Quality control tests were carried out with all wild populations, i.e. samples of 100 pupae for emergence and 100 pupae for flight ability from all wild and hybrid populations. For emergence 100 pupae were set up in small Petri dishes (9-cm-diameter). After five days following emergence the number of dead emerged males and females was recorded. Another sample of 100 pupae was used for flight ability. The pupae were placed within a 10 cm-high cylinder of acetate sheet lightly coated with talc in a 9 cm- diameter Petri dish. Flies could escape from these units by flight only and were recorded five days following emergence (Hooper, 1987; FAO/IAEA 2003).
>
Fertility and fecundity of hybrids between laboratory-adapted and wild olive flies
Following adult emergence, France-Wild and Democritus flies were maintained separately and sexed within the first three days after emergence as adults. Eleven day old (i.e. age of sexual maturity; Ahmad, unpublished data) virgin male and female flies from both strains were transferred to small cages (30 × 30 × 30 cm) with one side covered with screen netting and another side with wax-coated net as an oviposition medium. Thirty males and females were used for each of the following combinations: a) France-Wild ♂ × France-Wild ♀, b) Democritus ♂ × France- Wild ♀, c) France-Wild ♂ × Democritus ♀. Egg collection started the day after introducing the pairs and continued for eight days. Total volume of eggs, egg viability and recovery of pupae were recorded from each batch of eggs. The eggs collected from the different crosses were transferred to the larval diet on the same day at a density of 20 eggs/g of diet (Manoukas & Tsiropoulos, 1977). Pupae were collected 15 d following the date of seeding.
>
Table grapes as an alternative oviposition medium for wild olive flies
Wild olive flies collected from batches of infested olive fruits that originated from France, Spain, Italy and Croatia were used in this experiment in 2011.
Fifteen male and female flies from the France-Wild population were collected as single couples in separate plastic cups and allowed to complete copulation. Twenty-four h later each female (n = 15) was individually transferred to a small Plexiglas cage (9.5 × 4.5 × 4.5 cm) that contained one grape fruit for oviposition. Female and male flies for all other wild populations (Spain, Italy, and Croatia) were upon emergence pooled in a medium size Plexiglas cage (30 × 30 × 30 cm) that contained a bunch of grapes as an oviposition medium.
As the olive fly larvae were not able to complete their larval development inside the grapes (Ahmad, unpublished data), the second instar larvae (and unhatched eggs) were dissected out from the grapes with the use of a scalpel and soft camel brush, and transferred to the artificial larval diet where they were reared under standard laboratory protocols as described earlier.
>
Colony production from F1 - F5 generations of wild olive fly populations
Adult flies (F1) from all strains (France wild, Spain wild, Italy wild and Croatia wild) that were produced in experiment 2, were maintained in separate Plexiglas cages that had two or three sides covered with nylon mesh. Egg collection in each cage started on day 11 post emergence by introducing a wax panel bottle (diameter 16 cm, height 7.5 cm) as an oviposition device. The bottle had openings of 15 cm (diameter) × 6 cm (height) that contained panels of wax-coated nylon cloth. The number of collected eggs was recorded from each single cage for each generation and for each strain. Eggs collected from these wax bottles were transferred to the standard larval diet and standard rearing protocols for larvae development, pupae collection and adult fly maintenance were used for five generations.
The data of egg production, egg hatch, egg-pupa recovery and pupal weight for all strains and generations were analyzed using a one way ANOVA in Graph Pad Prism 6 (La Jolla, CA, USA). A post hoc Tukey's HSD test was employed for the analysis of pupal recovery performance of different cross-mating treatments.
>
Hybridization of wild and laboratory adapted strains
In the hybridization experiment using the wild strain from France and the laboratory-adapted strain from Greece, female flies began to oviposit thirteen to fourteen days after emergence. Females from the France-Wild ♂ × France-Wild♀ and the Democritus ♂ x France-Wild ♀ crosses deposited most of their eggs on the floor of the cages with only a few eggs collected from the wax panels. In the France-Wild ♂ × Democritus ♀ combination, females deposited most of the eggs on the wax panels (Table 1).
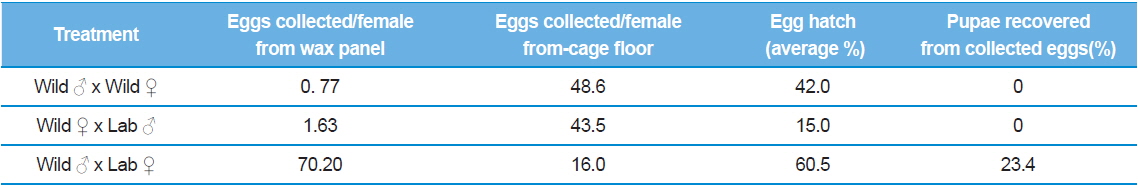
Production data from hybridization experiment with France wild strain and Greek lab strain. For each treatment 30♂ and 30♀ were used.
Data analysis revealed that total egg production, including eggs not oviposited on the wax panel, and egg hatch from all three treatments was not significantly different between strains: total egg production (F = 0.333, d.f.2,
The results of this experiment demonstrate that wild female olive flies have difficulty in ovipositing eggs under artificial rearing conditions as shown by the few offspring and zero pupae recovery rate obtained using wax panels as an oviposition device.
In a previous study with the Mediterranean fruit fly
This study suggested that introduction of wild males into the original colony is advantageous due to the positive effect of hybridity (hybrid vigor) and, subsequently, a greater production and quality profile in the subsequent progenies may be achieved. The benefits of introducing wild insects needs to be expanded towards other crucial characteristics of olive fly colonies and aspects such as male mating performance, dispersal ability and longevity in the field needs to be investigated to confirm the validity of this hypothesis.
>
Table grape as an alternative oviposition medium for wild olive flies
A total of 8.1 kg of infested olives were received from Croatia, France, Spain and Italy, from which a total of 1396 adults emerged. The sex ratios of all the strains were nearly equal (Table 2). The number of females that could be used for the oviposition experiment in grapes was very low and fluctuated between 5 and 45. Only 15 mated females from the France wild strain could be used individually for oviposition in grapes as the remainders of the emerged females died before reaching sexual maturity. . A total of 1211 eggs were collected in grapes over a period of 11 days. A total of 45 females from the Spain wild strain oviposited 890 eggs, whereas there were only 5 females from the Italy-wild and the Croatia-wild strain that produced 1030 and 663 eggs, respectively (Table 3). The experiment was only replicated once due to the unavailability of subsequent wild populations.
[Table 2.] Pupae collected, adult emergence and sex ratio of four wild olive fly populations

Pupae collected, adult emergence and sex ratio of four wild olive fly populations

Mean number of eggs/female and pupal recovery of grapes trial from all four wild population:
Wild female olive flies readily accepted grapes as an alternative oviposition substrate and larvae developed to the second instar, surviving inside the grapes for 3-4 d. Though we demonstrated the possibility to colonize wild populations of olive fly using grapes for egg collection, it is obvious that grapes can only be used as a temporarily egging device.
The oviposition behavior of wild female olive flies shows that surrogate fruits should be hard enough not to retract under the pressure of the ovipositor and still soft enough for the female fly to pierce a suitable hole within a certain time frame (Tzanakakis, 1989). It has been previously demonstrated that olive flies from a laboratory strain can also oviposit in tomatoes (Navrozidis & Tzanakakis, 2005). Another study demonstrated that oviposition could also occur in grape berries of several cultivars, less readily in some green or red-ripe tomatoes and green bell peppers, and with difficulty in spade pears (Tzanakakis, 1974). When in captivity and deprived of olives, a female olive fruit fly will attempt to lay in or on various objects with a smooth surfaces such as paraffin wax, glass, stretched Parafilm, or the floors of the cage. Therefore under captivity, is possible that eggs could be oviposited into fruits other than olives.
Previous studies have shown that tomatoes cannot successfully replace artificial larval diets for the complete larval development of olive fruit flies (Navrozidis, 2005). It is possible to replace an artificial diet only for short periods or for a small number of generations due to the unavailability or temporary shortage of some crucial ingredient (Navrozidis & Tzanakakis, 2005). This study showed that grapes can temporarily be used as an oviposition device when olives are not readily available and larvae can survive until the second instar. This method has value as it can greatly assist with the colonization of new strains, as this study has clearly shown.
>
Protocol for culturing the flies from F1 to F5 generations
For each strain, egging data from the wax bottles was collected from a single cage. Pupae were collected two weeks after seeding the eggs on the diet (Table 4) and all four wild strains showed similar production data. Production data varied considerably for the first 5 generations for each strain indicating that the adaptation process was slow (Table 4). Pupal weights data were collected from the first generation and the average were: Spain, 5.42 ± 1.52 mg, France, 4.36 ± 0.84 mg, Croatia, 5.35 ± 1.90 mg and Italy, 5.11 ± 1.63 mg. Average pupal weights were not significantly different amongst the strains (F=0.70,df=3,

Mean number of eggs per female and pupal recovery from generation F2-F5 was analysed by Anova: (F=2.67, df =2, P=0.094).
It has been reported that adult survival of the Mediterranean fruit fly was reduced during the first three generations of colonization on artificial diet (Leppla
The current study demonstrated that at the beginning of adaptation in laboratory conditions all wild females refused to lay egg on artificial wax panel and pupal recovery was low (Table 4). But, after a few generations, the wild females slowly adapted to using the wax-coated panels as an alternative egging device. Still, additional research is required to investigate other parameters of an efficient artificial rearing system.
In conclusion, this paper reports on the successful simultaneous colonization of 4 wild populations of olive fly and adapt them to artificial rearing conditions. To our knowledge, there are no reports in the literature that report on the successful colonizing wild olive fly populations in the last decades. The use of grapes as a temporary alternative oviposition substrate was instrumental in the adaptation process, due to the reluctance of wild females to use the wax panel as an oviposition device. The combination of using grapes and artificial larval diets in the first generation and wax panels for the subsequent generations was the basis of the protocol to allow successful colonization.



