Polar and northern Atlantic fish species survive in ice-laden or subzero environments. Antifreeze proteins and glycoproteins [AF(G)Ps], which are biological antifreezes, seem to be critical in terms of cold adaptation (DeVries and Wohlschlag, 1969; DeVries, 1971). AF(G)Ps, which were first discovered in the serum of Antarctic fish by DeVries and Wohlschalg (1969), are a group of proteins that lower the freezing but not the melting points of aqueous solutions (Jia and Davies, 2002). Solutes in the normal body fluids of temperate fish can depress the freezing point to -0.7℃, but polar and northern temperate fish must tolerate seawater at or below -1.9℃ (DeVries, 1971; Davies and Hew, 1990; Fletcher et al., 2001). AF(G)Ps further depress the freezing point by about -1℃, preventing fish fluids from freezing. Such freezing point depression is mediated by an adsorption–inhibition mechanism (Raymond and DeVries, 1977). AF(G)Ps adsorb (or bind) irreversibly to the surfaces of ice crystals in blood and inhibit their further growth, thereby lowering the blood freezing point. The temperature gap thus created, termed thermal hysteresis (TH), is a measure used to quantitatively assess the activity of AF(G)Ps.
Also, AF(G)Ps inhibit ice recrystallization (IR), a process whereby larger ice crystals grow at the expense of smaller crystals (Knight et al., 1984). IR is fatal to overwintering organisms (such as insects and plants) that experience fluctuations in temperature (Raymond and Fritsen, 2001; Raymond and Knight, 2003). IR inhibition likely protects membranes from freezing and helps organisms survive cold conditions (Janech et al., 2006). However, the IR property is not necessarily exploited by fish because they cannot tolerate freezing. In the present work, we regard both TH and IR inhibition as forms of “antifreeze activity.” The unique properties of AF(G)Ps impart many potential applications to such proteins, including their use as cryoprotectants in cell and tissue biobanks, in organ preservation, as food preservatives, as anti-icing agents, and in transgenic technologies seeking to impart cold-resistance (Davies et al., 1989; Hew et al., 1992; Wohrmann, 1996; Barrett, 2001; Ben, 2001; Bouvet and Ben, 2003; Harding et al., 2003; Fuller, 2004).
To date, five structurally diverse types of AF(G)Ps have been isolated from fish of the Antarctic, Arctic, and north Atlantic oceans: termed types I–IV and antifreeze glycoprotein (AFGP) (Davies and Sykes, 1997; Fletcher et al., 2001). We do not further discuss type IV AFP because its function has not been clearly established. Winter flounder and other fish from the east coast (50°N) of Canada express antifreeze proteins, which is important, as sea ice can form off this coast during winter. Nishimiya et al. (2008) extensively surveyed fish from coastal waters off Hokkaido and found that at least 50 species contained AF(G)Ps. This is not surprising; although Hokkaido is at 43.06°N, the coastal sea freezes during winter (Ice Information Center, 2015). Therefore, many fish in this area have evolved AF(G)Ps to facilitate adaptation to cold. Molecular analysis of AF(G)P genes suggested that novel antifreeze functions developed because of selective pressure (e.g., an extremely cold environment); the proteins do not share a common progenitor (Cheng, 1998; Cheng and Chen, 1999; Cheng et al., 2003; Cheng et al., 2006; Graham et al., 2013). Thus, AF(G)Ps constitute a textbook example of convergent evolution, supported by the fact that antifreeze activity (except that of AFGP) is generally absent from temperate and tropical fish. Functional AFGP genes have been described in fish of the suborder Notothenioidei (Order Perciformes) living in temperate waters off New Zealand; the ancestral gene likely originated in Antarctica (Cheng et al., 2003). Although the AF(G)Ps of polar and north-temperate fish have been well studied, those of temperate fish have not. We considered that further studies on antifreeze activity in fish living in ice-free and temperate oceans might yield insights into the distribution and evolution of AF(G)Ps. Hence, we sought antifreeze activity in temperate fish living off the coast of Jumunjin (37.89°N), Gangneung, Korea.
Nine fish species from the East Sea were purchased January 8, 2010, from the fish market of Jumunjin (37.89°N), Gangneung, Korea (Table 1). Blood, liver, and muscle were flash-frozen in liquid nitrogen on site and brought to the laboratory. Frozen blood was thawed on ice. Fifty μL amounts of precooled 1 × phosphate buffered saline (PBS) were added to the same volumes of blood, followed by thorough mixing using pipettes. These suspensions were frozen at -80℃ and then thawed on ice. This freeze–thaw step was repeated three additional times. The suspensions were centrifuged at 13,000 rpm and 4℃ for 10 min, and supernatants were collected and kept at -80℃ prior to analysis. Frozen liver and muscle samples were thawed on ice and washed with 1 × PBS to remove residual blood. Two hundred microgram volumes of each tissue sample were weighed, cut into small pieces, and ground in liquid nitrogen in a homogenizer. Each sample was next suspended in 200 μL precooled 1 × PBS. The freeze–thaw cycle described above was applied three times, with thorough vortexing between cycles. Each suspension was centrifuged at 13,000 rpm and 4℃ for 20 min, and the supernatant was harvested and stored at -80℃ prior to use.
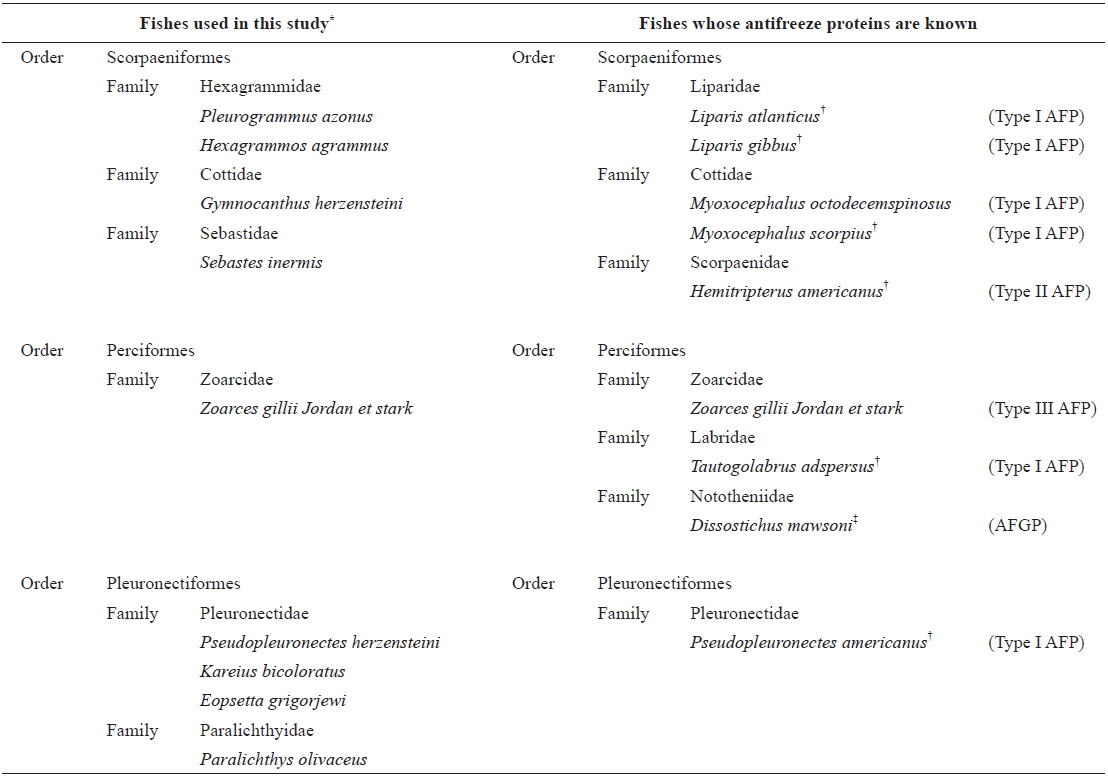
List of fish species used in this study and their north-temperate counterparts with functional antifreeze proteins. Habitats of these fishes are described in footnote
>
Thermal hysteresis (TH) activity
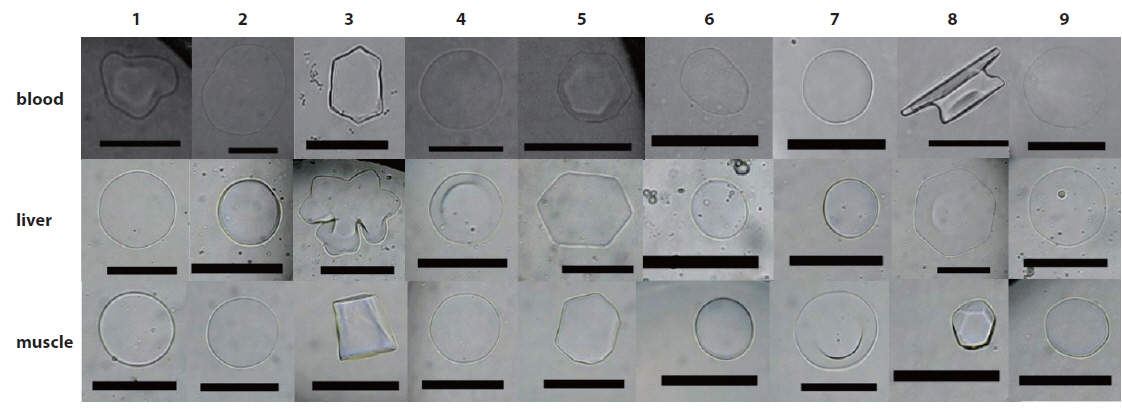
The thermal hysteresis (TH) and ice crystal morphology of each sample were examined using a nanoliter osmometer (Otago Osmometers, Dunedin, New Zealand), as described elsewhere (Lee et al., 2010). The osmometer was connected to a cold-well stage mounted on an Olympus CH-2 microscope equipped with a Canon Digital Camera. Briefly, about 1.5 μL of each sample was loaded into the osmometer, which was then completely filled with oil. The sample well was mounted onto the cold-well stage, rapidly frozen, and held below -20℃. This process created polycrystalline ice. The temperature was next slowly raised until only a single ice crystal remained; this was considered the melting point. The temperature was next lowered again at about 0.05℃/min, and morphological changes in ice crystals were examined. The temperature at which ice crystals began to grow rapidly was considered the freezing point. The TH value was calculated by subtracting the latter value from the melting point. All TH values were measured in triplicate. Ice crystal morphology was observed and recorded during antifreeze activity measurement; the same experimental method was employed.
>
Ice recrystallization inhibition assay
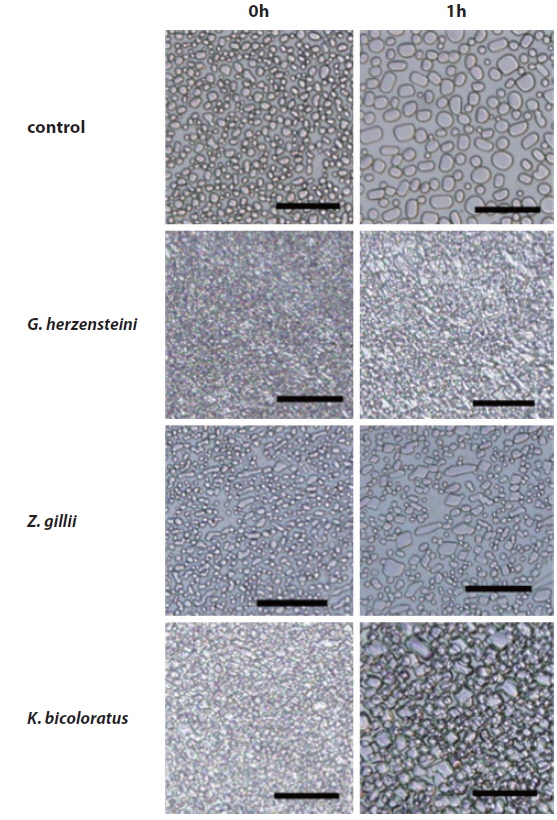
IR inhibition was measured using the method of Smallwood (Smallwood et al., 1999) with the aid of a Linkam TMHS600 cold stage (Linkam Scientific Instruments, Surrey, UK) mounted on an Olympus BX51 light microscope. Briefly, glycerol was added to each sample to a final concentration of 30% (v/v). Two 1-microliter amounts of each mixture were layered between two coverslips and loaded onto a silver block located within a THMS600 cold stage. The temperature was quickly lowered to -80℃ at a rate of 90℃/min, and this temperature was maintained for 10 min; fine ice crystals developed. The temperature was next increased to -6℃, and this temperature was maintained for 60 min, during which time changes in the ice crystals were noted; smaller ice crystals grew into larger crystals.
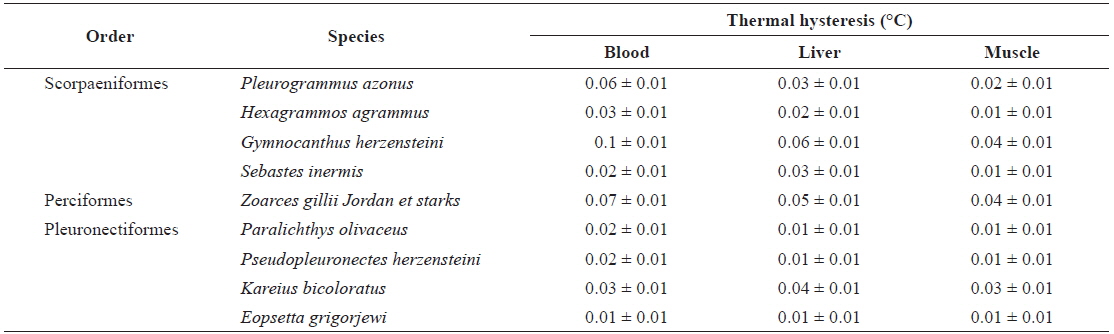
All fish species studied were caught off the coast of Jumunjin (Table 1), where more than 77 fish species can be found (Yang et al., 2012; Lee et al., 2013; Sohn et al., 2014); all were caught on the morning of the sampling day. The Korea Ocean Observing and Forecasting System reported that the average temperature of surface seawater in the Jumunjin area 1 week before and 1 week after the date of purchase was about 9℃, well above the freezing point of seawater (http://sms.khoa.go.kr/koofs/eng/observation/obs_real_map.asp). Also, sea ice does not form in this area even in winter. Our fish samples comprised three orders, six families, and nine species (Table 1). Four were of the order Scorpaeniformes, one of the order Perciformes, and four of the order Pleuronectiformes. The rightmost column of Table 1 lists fish species exhibiting antifreeze activity expressed by relevant genes (Graham et al., 2013). As TH and changes in ice crystal morphology are indicative of the presence of antifreeze proteins, the THs of blood, liver, and muscle samples were measured using a nanoliter osmometer, and we simultaneously observed changes in ice crystals. The detection limit of the osmometer was 0.01℃. Intriguingly, of the nine fish species tested, four exhibited discernible blood TH values; these were P. azonus,
[Table 2.] Thermal hysteresis of nine fishes used in this study
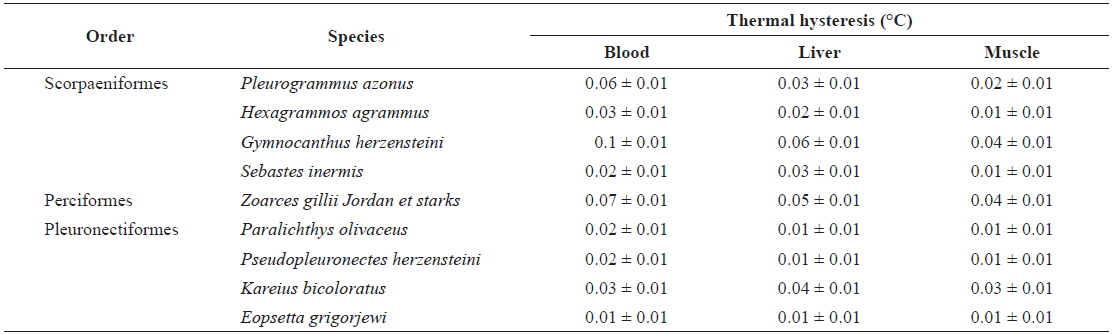
Thermal hysteresis of nine fishes used in this study
>
Ice recrystallization inhibition
IR inhibition by