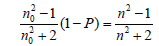The rapidly developing microelectronics industry has shown a strong demand for high performance materials to improve device performance [1,2]. Nanoporous silicate thin films are considered as important materials for advanced electronics. The formation of air-filled nanopores is a key technology for preparing insulating materials [3-12]. Polymethylsilsesquioxane (PMSSQ), with the empirical formula of (CH3-SiO1.5)n, is a potential candidate for low dielectric spin-on glass [13]. It has the characteristics of low dielectric constant ranging from 2.7 to 2.9, high thermal stability, low moisture uptake, good adhesion to metal, and excellent mechanical properties [11,14]. In addition, star-shaped macromolecules, such as hyperbranched poly(ε-caprolactone) (PCL), are generally used as an organic template to generate nanometer-sized pores in the matrix polymer [11,15].
The organic templates dispersed in the polymer matrix are required to completely decompose at a relatively low temperature range (200℃ ~ 400℃), and after the thermal calcination process, the derived nanopores need to be well distributed as a closed-cell structure in the polymer matrix. The air-filled nanopores in a thin film can be generated by a method that disperses organic templates into an insulating matrix material, and decomposes them at elevated temperature. The mechanical strength of an insulating material will undoubtedly decrease, and the size and distribution of the as-made pores cannot be uniform, due to the organic templates, which leave pores behind from the thermal decomposition. Moreover, if the pores agglomerate, the insulating material may break down at low voltage. Therefore, the thermal decomposition and dispersibility of the organic templates in matrix polymers, and control of the nanopore generation, should be suitably considered for the structural design of organic templates.
In order to effectively generate and thoroughly distribute nanopores in the whole matrix film, the organic templates should be completely dispersed in matrix polymers, and the micro-phase separation of the templates and the matrix polymers should be minimized, during the curing of matrix polymers. In this work, we prepared organosilyl-modified and star-shaped poly(ε-caprolactone) (m-PCL) as organic templates, to be closely compatible with PMSSQ. This organic template was incorporated in PMSSQ, to make a homogeneous composite. The organic templates and PMSSQ were chemically linked to each other during the curing process. When heated to elevated temperature, the thermally labile organic templates decompose, and subsequently leave the isolated air-filled pores behind. We investigated the refractive indexes of the resulting porous PMSSQ films.
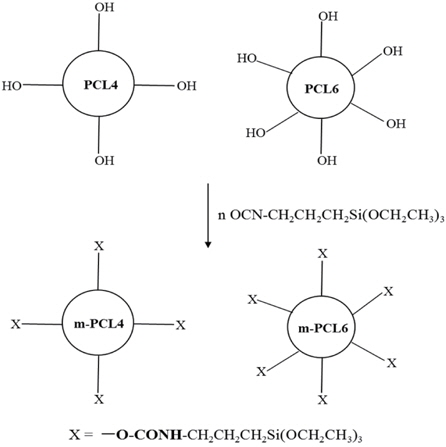
All chemicals were reagent grade, and were used without further purification. Polymethylsilsesquioxane (PMSSQ, GR650F™) was supplied by Techneglas Company (USA) as a resin, with a molecular weight of 10,000. The polymer structure is based on an alternating silicon-oxygen network with methyl pendant groups, as well as hydroxyl and ethoxy functional groups. More detailed information about PMSSQ is available elsewhere [13,16,17]. As shown in Fig. 1, the organic template as a pore generator was based on the star-shaped poly(ε-caprolactone) (PCL), which bears an aliphatic ether core and four (or six) polyester arms with hydroxyl end groups, and which is synthesized by the standard procedure [6,10,18]. In addition, the triethoxysilyl-terminated analog (m-PCL) was also prepared, as described in our previous work [6,10]. Molecular weights of as-synthesized PCLs were measured by gel permeation chromatography, based on polystyrene standards.
Both PMSSQ and organic templates (m-PCLs) were completely dissolved in methyl isobutyl ketone (MIBK) at 5 wt.%, resulting in a homogeneous solution. The compositions of m-PCLs were adjusted from 0 to 50 wt.%, with respect to PMSSQ. The asprepared composite (m-PCL + PMSSQ) solutions were filtered through PTFE-membranes with a pore size of 0.20 μm, before use.
The composite solutions with different compositions were spin-coated onto the pre-cleaned Si wafers, and subsequently dried at 50℃ for 1 h under nitrogen atmosphere. The dried composite films were thermally cured at 2℃/min up to 200℃, and held at that temperature for 100 min, before natural cooling. During this curing process the PMSSQ was vitrified via a bulk polymerization, and finally the PMSSQ and organic templates were chemically linked to each other in films. To make porous PMSSQ films, the fully cured and organic-templated PMSSQ were further treated at 400℃ for 1 h, resulting in the thermal decomposition and elimination of the organic templates.
The thermal decomposition of organic templates was investigated under nitrogen flow of 80 mL/min on a thermogravimetric analyzer (EXSTAR 6000 TG/DTG, Seiko), with a heating rate of 2℃/min. The thicknesses and refractive indices of organic-templated PMSSQ composites and the resulting nanoporous PMSSQ films were measured using a spectroscopic ellipsometer (J. A. Woollam Company, Model M-44), which was equipped with a Xe lamp light source (wavelength 400 ~ 800 nm).
Star-shaped poly(ε-caprolactone)s (PCLs) are widely applied as pore generators (organic templates) in nanoporous material research [3-10,17]. These templates used in this work bear aliphatic ether cores, and four (or six) polyester arms. The PCL synthesis and its modification to triethoxysilyl-terminated PCLs (m-PCLs) follow the previous methods [6,10].
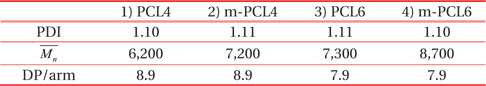
Table 1 summarizes the molecular information of the organic templates. Terminal triethoxysilyl groups of m-PCLs improve the dispersibility of m-PCLs in the PMSSQ matrix, because the terminal groups are chemically similar to PMSSQ. Moreover, PMSSQ and m-PCLs can make chemical bonds with each other during the curing process. As a result, the introduction of triethoxysilyl groups into PCLs suppresses the phase separation of the m-PCLs and PMSSQ [3-7,10,17]. The nanopore structures could be made and evenly distributed in the resulting PMSSQ film, via curing and calcination. The smaller sizes and uniform distribution of the pores significantly improve the structural stability of the film [3-10].
[Table 1.] Molecular information of the as-prepared organic templates.
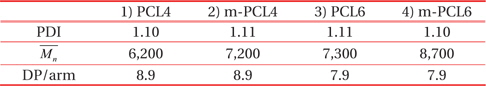
Molecular information of the as-prepared organic templates.
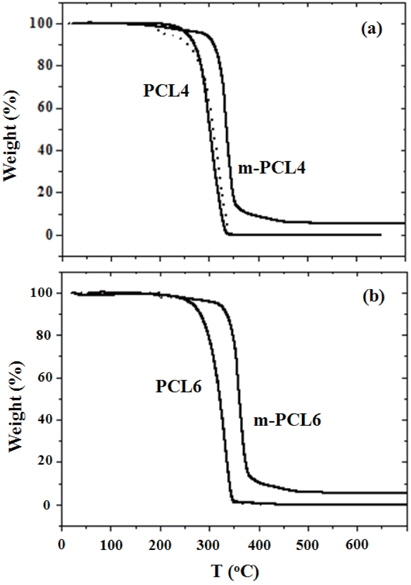
To investigate the applicability of as-prepared organic templates as pore generators in the PMSSQ thin films, thermal degradation behaviors of the PCLs were studied under nitrogen with a heating rate of 2℃/min by thermogravimetric analysis. If the PCLs decompose during the PMSSQ curing, they will not work properly as pore generators. As presented in Fig. 2, both PCL4 and 6 began to decompose over 200℃, and were fully decomposed below 400℃. The dashed lines of Fig. 2 indicate the TGA traces of PCL4 and 6 during thermal processes of 200℃ for 100 min, and further 400℃ for 60 min. As compared to solid lines, the whole traces overlapped well with each other. Both m-PCL4 and 6 began to decompose at ~50℃ higher temperatures than their hydroxy-terminated analogs (PCL4 and 6).
This elevation of decomposition temperature can be explained by the thermal degradation of the PCLs via an unzipping mechanism [18]. Because the triethoxysilyl end groups of m-PCL4 and 6 are thermally more stable than the terminal hydroxyl groups of PCL4 and 6, the thermal decomposition of m-PCLs by the unzipping mechanism was much delayed. We could choose the temperatures for vitrification of PMSSQ and decomposition of the templates, from these TGA results of the organic templates: heating at 2℃/min to 200℃, and held for 100 min at that temperature for the curing of the PMSSQ, and further treatment up to 400℃ with the same heating rate, and held for 60 min at that temperature for the template decomposition. All the thermal processes were performed under nitrogen flow.
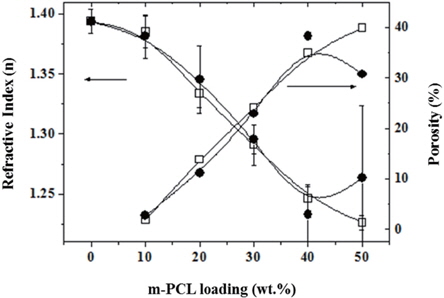
The refractive indexes of porous PMSSQ films were measured by spectroscopic ellipsometer equipped with Xe lamp light source (λ = 400~800 nm). Ellipsometry is an easy and precise method to obtain the thickness and refractive index of thin film, such as spin-on-glass. The porosity in a porous PMSSQ film can be estimated from the changes of the refractive indexes by the Lorentz-Lorenz relationship [19,20], as follows:
where, n0 and n are the refractive indexes of the cured and calcined PMSSQ films, with and without initial template loading, respectively; and P represents the porosity in the porous PMSSQ film. Figure 3 shows the refractive indexes and the porosities of the porous PMSSQ films prepared from m-PCLs-templated PMSSQ composites. The refractive indexes of the porous films decreased with the increase of initial m-PCL loadings, resulting in increasing porosities of the films. The organic templates added to the PMSSQ composite films completely decomposed at elevated temperature, and remained voids in the films [3-13,16]. In addition, there are many previous studies for the size and structure of the pores generated in PMSSQ films [3-10]. The decrease of refractive indices was distinctly caused by the generation of pores in the films. The refractive indices of the porous PMSSQ films reciprocally decreased with increase of the initial template loading, regardless of whether the template was m- PCL4 or 6. When the porous PMSSQ film was prepared from the PMSSQ composite film with initial m-PCL loading of 50 wt.%, its refractive index re-bounced with a large error range. This result demonstrated that the networked wall of the porous PMSSQ film was unstable, and partially collapsed. Although the hydroxyl end groups of PCLs were chemically modified with triethoxylsilyl groups to inhibit the phase separation between PMSSQ and the templates, the phase separation somewhat progressed in m- PCL6-templated PMSSQ film, and some macropores were made for the calcination of PMSSQ film, resulting in the collapse of the porous film [3].
The dielectric constant (ε) of a material can be simply estimated by the Maxwell relationship, ε = n2 [21]. The ε values of the final porous films in this work were estimated in the range of 1.440 ~ 1.960, according to the initial template loadings.
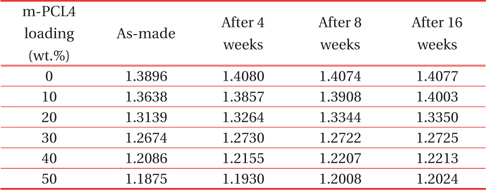
Table 2 shows the refractive indices that we recorded in the porous PMSSQ films prepared from PMSSQ composites with m-PCL loadings of 0~50wt.%. This experiment was conducted to investigate whether the refractive indices of the porous PMSSQ films changed with the ambient moisture.
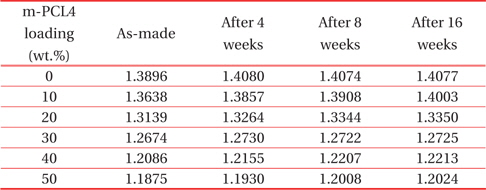
The Refractive indices with the lapse of time on the porous PMSSQ films prepared from m-PCL4 templated PMSSQ.
One serious concern of porous dielectric materials is the increase of dielectric constant caused by moisture adsorption. In practice, many kinds of porous silicate aerogels and xerogels were modified with hydrophobic surfaces, to reduce moisture uptake [22]. The refractive indices slightly increased, regardless of the template loadings. This result indicates that the porous PMSSQ films are strongly hydrophobic and resist moisture uptake. Similar results were obtained in the case of porous films prepared from m-PCL6-templated PMSSQ.
All of the samples were fully cured and calcined to prepare the porous PMSSQ films. As-prepared porous PMSSQ films were stored at an ambient condition.
m-PCL4 and 6 were modified with triethoxysilyl groups to chemically bind with PMSSQ, and to resist phase separation in PMSSQ films. The thermogravimetric results showed that m-PCLs completely decomposed in the temperature range of 200 ~ 400℃. The refractive indices of the as-made porous PMSSQ films continuously decreased with the increase of the template loadings in the PMSSQ composites, because the refractive index was strongly dependent on the porosity in the film. As m-PCLs were loaded higher in the PMSSQ composite, more pores were generated in the calcined PMSSQ films. When a considerable amount of pores were made in the calcined film, the porous film was susceptible to collapse, because of the weak networked structure of the porous film. The fact that the refractive index re-bounced in the porous film prepared from an m-PCL6 loading of 50 wt.% indicates that some pore structures collapsed, and the resulting film was densified. In addition, the refractive index of the porous PMSSQ films slightly changed after a long time in an ambient condition, because of their intrinsic hydrophobic characteristics.