Graphene, a one atom thick, 2D lattice of sp2-bonded carbon atoms [1-3], has received significant attention from researchers all over the world owing to its excellent optical, mechanical and electrical properties [4-9]. Graphene sheets are held together by the van der Waals force of attraction. However their inherent tendency towards restacking limits their applications. To achieve stable dispersion of graphene sheets in a polymer, it might be fruitful to incorporate polar groups such as carboxyl, hydroxyl via the covalent/non-covalent functionalization approach. Stable aqueous or organic suspensions of graphene have been achieved via chemical modification and/or oxidation-reduction of graphite oxide [10-12]. However, the functionalization of graphene via chemical oxidation-reduction methods requires the involvement of strong and hazardous acids which in turn destroy the sp2 carbon network and change the sp2 hybridized graphene into an sp3 network with significantly reduced properties.
This report has investigated sugar azide for the edge-functionalization of graphene via covalent bond formation without disrupting the graphene structure. Sugars are very rich in hydroxyl groups and were therefore chosen as a promising material for functionalization. The aim of our study was to create additional –OH groups on the graphene nanoparticle (GnP) in order to achieve a stable dispersion in a polar organic solvent without disrupting the basal plane of graphene. The additional polar groups are also beneficial as sites for covalent bond formation with an epoxy matrix and thus can contribute to enhancing the themo-mechanical properties of an epoxy/graphe ne nanocomposite.
GnP (size: 15 μm, thickness: 5-7 nm, surface area: 120-150 m2/g) was supplied by XG Sciences, USA. 2,3,4-Tri-O-acetyl-β-D-xylopyranosyl azide, known as sugar azide was purchased from Sigma Aldrich. Epoxy (Epon 828) was purchased from Miller Stephenson. Ethyl acetate (EtOAc), sodium methoxide (NaOMe) and metaphenylene diamine (MPDA) were purchased from Sigma Aldrich.
2.2. Functionalization of GnP using Sugar Azide
A weighed amount of GnP was mixed with 1,1,2,2-tetrachloroethane (TCE) and the mixture was ultrasonicated for 10 min. The solution was then purged with nitrogen and preheated to 150°C. Subsequently, sugar azide was dispersed in TCE and the solution was added drop-wise to the reaction mixture over a period of 20-30 min. The temperature was maintained at 150°C for 1 h, after which the product was cooled to room temperature under flowing nitrogen. EtOAc was added into the cooled reaction mixture. The crude product will filtered on 0.22 μm teflon membrane and washed copiously with EtOAc and methanol (MeOH). The residue left on the filter paper was dried under vacuum at 50°C overnight and this product was labelled as azide-functionalized GnP (SAMG-I). SAMG-I was mixed with ethanol (EtOH) followed by the addition of NaOMe (30%) in MeOH. The reaction mixture was stirred under nitrogen for 24 h at room temperature and then was slowly transferred into distilled water drop by drop. The basic mixture was then neutralized with a few drops of HCl (37%). The deacylated product was then filtered on 0.22 μm teflon membrane and repeatedly washed with distilled water and EtOAc. Finally, the (SAMG) was dried at 50°C under vacuum overnight.
2.3. Fabrication of epoxy/GnP composite
Epoxy was mixed with SAMG using a Flack-tek speed mixer for 2 min at 2500 rpm followed by ultrasonication for 30 min at 60 watts. The solution was degassed in vacuum for 5 min. MPDA was added to the mixture and again mixed using a Flacktek speed mixer for 2 min at 2000 rpm. The entire mixture was degassed for 5 min at 70°C. The solution was poured into a silicone mold to make coupons. The mixture was cured at 75°C for 2 h and at 125°C for another 2 h. The coupons were polished prior to testing.
Raman spectra were recorded in the range of 500 to 3500 cm-1 in a LabRAM ARAMIS confocal Raman microscope using a He-Cd laser beam with a wavelength of 532 nm. X-ray photo electron spectroscopy (XPS) was performed with a Perkin Elmer Phi 5600 ESCA (Kratos Analytical Ltd., UK), using an unmonochromatized Mg-Kα Xray source at a take-off angle of 45°. Dynamic mechanical analyses (DMA 800, TA instruments) of the samples was conducted from room temperature to 200°C in the single cantilever mode at a heating rate and frequency of 3°C/min and 1 Hz, respectively. Thermogravimetric analysis (TGA) of the neat cured epoxy and its composites with GnP was carried out using TGA 500 (TA instruments, USA) with a heating rate of 10°C/min from room temperature to 700°C in air. Flexural properties of the samples were tested using a United Testing System (UTS, SFM-20 load frame) with a 100 pound load cell according to ASTM D790. The morphology of the fracture surface was observed by Scanning electron microscopy (SEM; Carl Zeiss Varible Pressure SEM EVO LS25). Samples were sputter-coated with tungsten prior to their SEM observation.
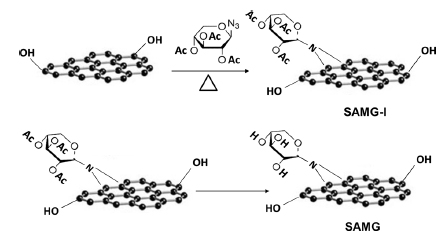
Sugar azide, a highly reactive substance, forms nitrenes by thermal or photocatalytic decomposition. The in-situ formed nitrenes react with the double bonds at the edges of GnP to form the azide adduct. Finally, the -OAc groups were hydrolyzed to form hydroxyl terminated azide modified GnP (SAMG). The pathway of SAMG formation is shown in Scheme 1.
4.1. Characterization of functionalized graphene (SAMG)
4.1.1. X-ray photo electron spectroscopy
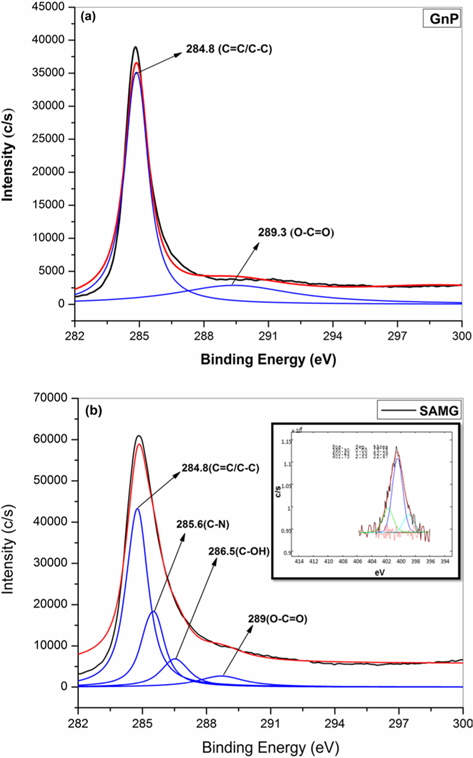
Fig. 1a and b show the C-1s XPS patterns of GnP and SAMG, respectively. The spectrum of GnP can be deconvoluted into two different peaks. The peaks at 284.8 and 289.3 eV have been assigned to the C–C/C=C in the aromatic rings, and O–C=O groups, respectively [13-15]. However, the C-1s XPS patterns of SAMG have been deconvoluted into four different peaks. Two new peaks at 285.6 and 286.5 eV have been assigned to C-N and C-OH groups, respectively. The N-1s spectrum of SAMG is shown in the inset of Fig. 1b. The well-defined peaks at 398.9 and 401.7 eV clearly suggest the existence of bonded nitrogen in SAMG.
4.1.2. Atomic force microscopy
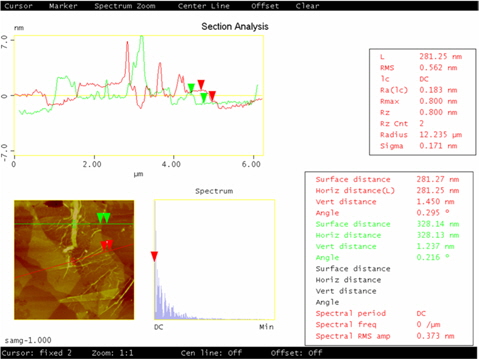
Atomic force microscopy (AFM) characterization allows the number of graphene layers to be identified. The AFM image of SAMG is shown in Fig. 2. The thickness of SAMG, measured from the height profile of the AFM image, is about 1.45 nm. The thickness of the SAMG sheets is drastically reduced compared to neat GnP (5-7 nm) indicating the successful exfoliation of graphene layers upon functionalization. However, the difference in height value of the SAMG compared to the theoretical value of a monolayer of graphene is due to 1) the presence of the oxygenated moiety as a result of functionalization and 2) the resulting few layers (4-5) of graphene.
4.1.3. Thermogravimetric analysis
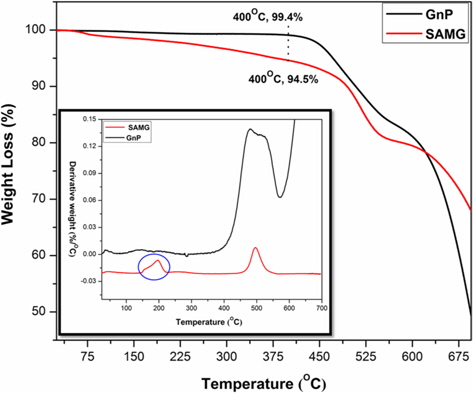
TGA thermograms of GnP and SAMG are shown in Fig. 3 and the derivative plot (DTG) is shown in the inset of Fig. 3. Neat GnP is quite stable up to 450°C. The weight loss after 450°C is due to degradation of the carbon skeleton of GnP. On the other hand, SAMG exhibited almost 5% weight loss at 400°C. The DTG curve showed that the thermal history of SAMG involves a two-step degradation process. The initial weight loss in the temperature range of 150°C-210°C corresponds to a combined weight loss due to degradation of the azide and oxygenated groups, which were formed upon functionalization. The final weight loss at around 500°C is due to cleavage of the carbon skeleton of GnP.
4.1.4. Raman spectroscopy
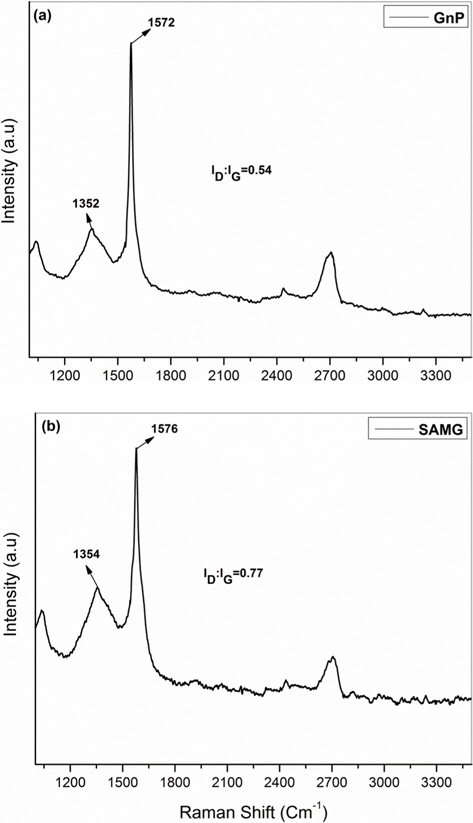
Raman spectroscopic analysis is an important tool for defining the quality of graphene. Fig. 4a and b show the Raman spectra of the GnP and SAMG, respectively. The Raman spectrum of the GnP has a characteristic D band at 1352 cm-1 (the breathing mode of C-sp2 atoms in the rings, which is related to disorder within the structure) and a G band at 1572 cm-1 (the in-plane bond stretching motion of C-sp2 atoms) [16-19]. However, the Raman spectrum of the SAMG (Fig. 4b) showed an increment in the ID:IG ratio, from 0.54 to 0.77, which can be attributed to an enhancement in defect concentration due to functionalization, i.e., the conversion of π-bonded C-sp2 carbons to C-sp3 ones. Moreover, the higher ID/IG ratio signifies a higher degree of covalent functionalization [20]. However, the shape of the ‘G’ band at 1579 cm-1 was observed to be sharp, suggesting that the functionalization occurred at the edges without disrupting the basal plane of the GnP.
4.1.5. Scanning electron microscopy
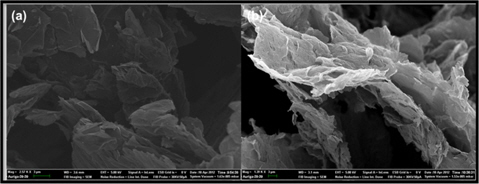
SEM micrographs of neat GnP and SAMG are shown in Fig. 5a and b. The surface of the GnP was smooth and clean (Fig. 5a). However, while extensive functionalization did not affect the basal plane or the flaky structure of GnP, the surface of the SAMG became rough and wavy after functionalization (Fig. 5b).
4.2. Thermo-mechanical performance of epoxy/SAMG composite
4.2.1. Thermogravimetric analysis
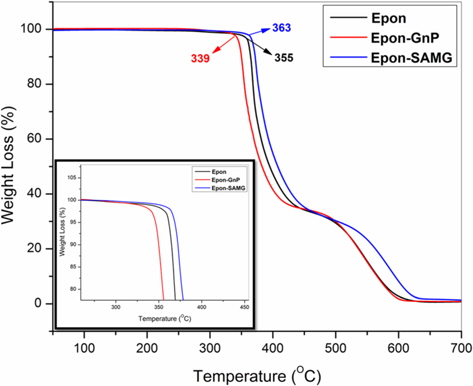
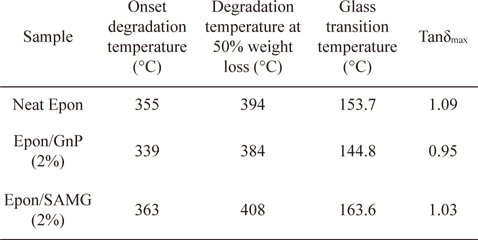
The thermal stability of neat epoxy and its nanocomposites is shown in Fig. 6. The onset degradation temperature of neat epoxy appears at 355°C. However, the onset degradation temperature was reduced to 339°C for the epoxy/GnP (2 wt%) nanocomposite. At 50% weight loss, the degradation temperatures of neat epoxy, epoxy/GnP (2 wt%), and epoxy/SAMG (2 wt%) nanocomposites were observed to be 394°C, 382°C, and 408°C, respectively. The decrease in the thermal stability of the epoxy/GnP (2 wt%) nanocomposite was probably due to poor interfacial adhesion between filler and matrix resulting from the inhomogeneous distribution of GnP particles throughout the matrix. On the other hand, functionalization resulted in the creation of polar groups which in turn facilitated the homogeneous distribution of SAMGs throughout the matrix. Homogeneously distributed functionalized graphene layers could reduce the emission of small gaseous molecules resulting in delayed thermal degradation of the nanocomposites compared to neat epoxy and the epoxy/GnP composite.
4.2.2. Dynamic mechanical analyses
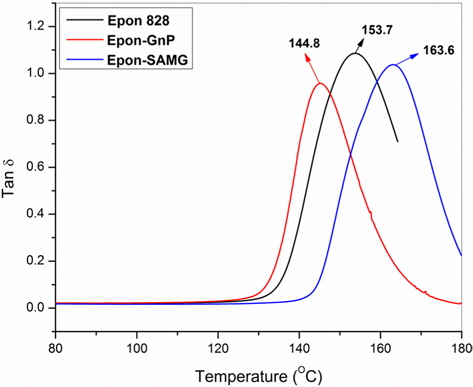
The variation of loss tangent (Tanδ) as a function of temperature is shown in Fig. 7. Tanδ is the ratio of the loss modulus to the storage modulus and is very sensitive to structural transformations. Tanδ for neat epoxy appears at 153.7°C, which is assumed to be the glass transition temperature (Tg) of the neat epoxy. However, in the presence of GnP, the Tg has shifted to a lower temperature region (144.8°C) suggesting partial immiscibility between the matrix and filler, leading to the poor interfacial adhesion. However, functionalization resulted in a substantial increment in Tg, suggesting the formation of better Funcadhesion and a strong interface between SAMG and epoxy. Superior adhesion allows better stress transfer from filler to matrix. The details of the thermal stability and Tg’s of neat epoxy and nanocomposites have been summarized in Table 1.
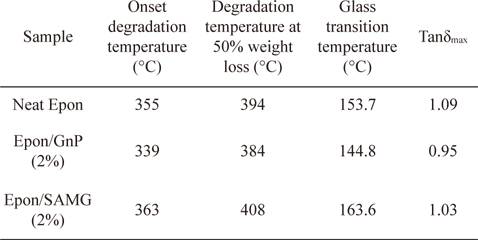
Thermal stability and dynamic mechanical properties of neat epoxy and its nanocomposites with GnP
4.2.3. Scanning electron microscopy
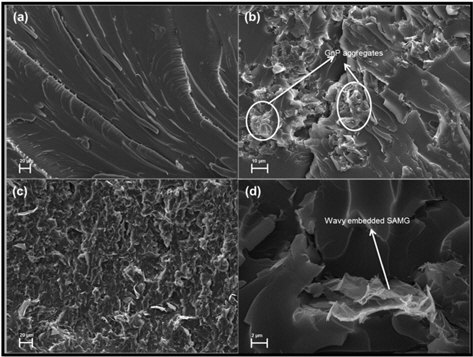
Fractured surfaces (from flexural tests) of neat epoxy and epoxy nanocomposites were investigated by SEM and the micrographs are presented in Fig. 8a-d. The fracture surfaces of neat epoxy were smooth and clean (Fig. 8a). The epoxy/GnP (2 wt%) nanocomposite displayed the clear formation of GnP aggregates. The aggregates were very poorly distributed in the matrix, and this was responsible for its poor thermal and mechanical properties (Fig. 8b). On the other hand, functionalization caused a more homogeneous distribution of SAMGs throughout the matrix (Fig. 8c). The high magnification SEM image of epoxy/SAMG (2 wt%) nanocomposite (Fig. 8d) further shows that the wavy SAMG particles were embedded into the matrix, suggesting better wetting and stronger bonding between epoxy and SAMG, which in turn caused an improvement in thermal and mechanical properties to a reasonable extent.
4.2.4. Flexural properties
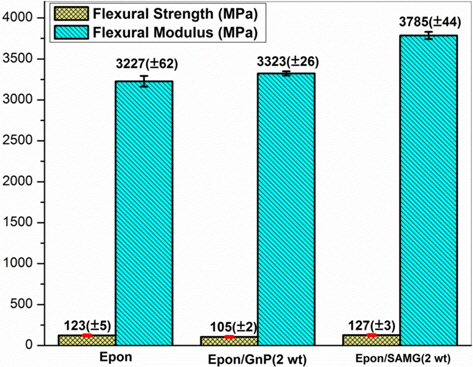
Fig. 9 represents the effect of functionalization on the flexural modulus and strength of GnP/epoxy nanocomposites. Functionalization resulted in an 18% improvement in flexural modulus as compared to neat epoxy. Functionalization of GnP results in: 1) creation of groups which can react with the epoxy matrix and 2) prevention of restacking of GnP nanoparticles, which helps produce a more homogeneous distribution. The flexural strength of the epoxy/SAMG (2 wt%) nanocomposite system showed a negligible increment.
Nitrogen-linked graphene was synthesized using sugar azide via the formation of nitrene as an intermediate. Polar groups created via functionalization facilitated hybrid nanocomposite formation with improved thermo-mechanical properties. Functionalization of graphene was verified with spectroscopic studies and thermal analysis. The SAMG nanocomposite showed a reasonable increase in glass transition temperature as compared to neat matrix and the epoxy/GnP composite, suggesting strong interfacial adhesion between the functionalized graphene and the epoxy. Morphological observation revealed that the functionalized GnPs were rough, wavy and were embedded into the matrix, confirming the strong adhesion between filler and matrix. The flexural modulus was 18% higher compared to neat epoxy.