Patients usually take several drugs for the treatment of one or more health problems at the same time. Metabolic drug-drug interactions occur when a drug inhibits or induces the activity of a drug-metabolising enzyme, which catalyses the metabolism of a concomitantly administered drug. Interaction however is not limited to drugs but includes also other supplements that patients may be taking at the same time including vitamins, minerals, food supplements and herbal remedies. Herbal medicines have been used worldwide since ancient times. Archeological evidence and pollen analysis from a Neanderthal burial site in modern day Iraq indicate that the use of medicinal plants may be dated back to 50,000 B.C. (Solecki and Shanidar, 1975). An estimated one third of adults in the United States use alternative therapies, including herbs (Kauffmanet et al., 2002; Shi and Klotz, 2012). It has also been reported that 20% of the general population in the United Kingdom used complementary or alternative medicine in the course of a year (Zhou et al., 2004). There is a common belief among the general public that herbal preparations are “good for humans” as they are “all natural” (Solecki and Shanidar, 1975). However, they are complex mixtures of bioactive entities that may or may not provide therapeutic activity. Usually, the
COMMON MECHANISMS OF INTERACTION
The clinical importance of any drug interaction depends on several factors, including the condition of the patient, the drugs administered, the route of administration, the environment, the therapeutic index, as well as the timing of administration of the drugs (Kremers, 2002). The possible causes of drug interactions can be summarized as follows: ■ Competition for gastrointestinal absorption■ Binding to plasma proteins■ Binding to transport proteins■ Pharmacodynamic interactions at receptor level■ Inhibition of metabolism■ Induction of metabolism■ Competition for active renal excretion
The gastrointestinal absorption of drugs may be affected by concurrent use of other agents due to the large surface area of the tract upon which the drug can be absorbed, bind or chelate, or alter in term of gastrointestinal motility. The mechanisms by which drug interactions alter drug distribution include competition for plasma protein binding and displacement from tissue binding sites. And this binding can increase the free concentration (and thus the effect) of the displaced drug in plasma. Pharmacologic interactions between agonists and antagonists happen at specific receptor sites, resulting in the effects of drugs being blocked or changed. From the drug metabolism perspective, one notable system involved in metabolic drug interactions is the enzyme system comprising the cytochrome P450 (CYP) monooxygenases. This system may be affected by either enzyme induction or enzyme inhibition. For instance, drug A induces the body to produce more of an enzyme which metabolises drug B. This reduces the effective concentration of drug B, which may lead to loss of its effectiveness. On the other hand, drug C inhibits the production of the enzyme metabolising drug B, thus an elevation of drug B occurs possibly leading to an overdose. The renal excretion of active drug can also be affected by concurrent drug therapy, thus altering serum drug level and pharmacologic response.
Herbal remedies are mixtures of more than one active ingredient. Hence, the likelihood of drug-herb interaction is theoretically higher than drug–drug interaction because synthetic drugs usually contain single chemical entities. The interaction is likely to follow the same pharmacokinetic and pharmacodynamic mechanisms as seen in drug–drug interactions (Izzo et al., 2002; Shi and Klotz, 2012). Drug–herb interactions can occur when herbs and drugs are coadministered and the herbal preparation (one or more components) modulates the metabolism of the drugs. Moreover herbal components can also be metabolised by CYPs or other drug-metabolising enzymes thus further complicating the mechanism.
CLINICAL RELEVANCE OF DRUG-HERB INTERACTIONS
The metabolism of a drug can be altered by another drug or foreign chemical and such interaction can often be clinically significant. Inhibition of the metabolism of a drug will lead to an elevation of its concentration in tissues, which might result in various adverse reactions, particularly for drugs with low therapeutic indices. For example, fluconazole, a potent inhibitor of an important human CYP isoform, CYP2C9, resulted in reduction of approximately 70% of the metabolic clearance of S-warfarin, leading to organ bleeding in patients (Black et al., 1996). Inhibition of CYPs has also led to the withdrawal of several marketed drugs during the past decades. The calcium channel blocker mibefradil (Posicor) used for the treatment of hypertension and chronic angina pectoris was voluntarily withdrawn by Roche from the US market in 1998 because it was a potent mechanism-based enzyme inhibitor that significantly increased the plasma concentration of other cardiovascular drugs (such as propranolol and verapamil) to toxic levels (Mullins et al., 1998a). The non-sedating antihistamines terfenadine and astemizole were withdrawn from the US market because of remarkable metabolic inhibition by other drugs leading to life-threatening arrhythmias (Dresser et al., 2000). In addition, the effectiveness of some prodrugs may be modulated by changes in CYP activities, for instance, CYP inhibition may result in a decrease in the formation of the active species, possibly leading to therapeutic failure due to lack of efficacy of the drug. Tamoxifen, a selective estrogen receptor modulator, could significantly reduce the conversion of the prodrug losartan to its active carboxylic acid metabolite (E-3174) by inhibiting the activity of hepatic CYP2C9 in breast cancer patients, hence affecting the efficacy of the angiotensin II receptor antagonist (Boruban et al., 2006).
The interaction problem applies similarly to herbal remedies. Although herbs are often promoted as natural and therefore harmless, they are not free from adverse effects. A recent observational study indicates that herbal supplements are associated with adverse events that include all levels of severity, organ systems, and age groups (Palmer et al., 2003). A relevant safety concern associated with the use of herbal remedies is the risk of interaction with prescription medications. This issue is especially important with respect to drugs with narrow therapeutic indices, such as warfarin or digoxin (Shi and Klotz, 2012). In clinical practice, polypharmacy is common, in addition to medicines prescribed by their physicians; patients may be taking various over-the-counter medications, vitamins, herbs and food supplements concurrently. All ingested substances have the potential to interact, resulting in various clinical problems such as therapeutic failure, drug adverse effect and toxicity, and altered patient response to therapy.
COMMONLY USED HERBS IMPLICATED IN DRUG INTERACTION
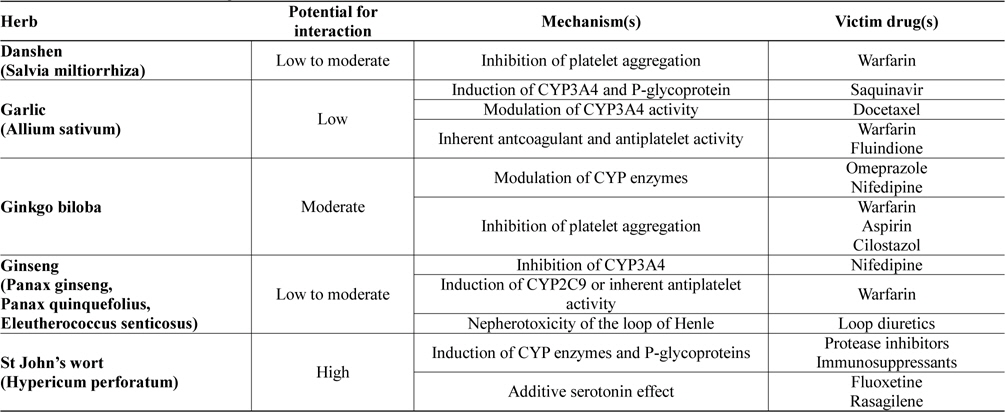
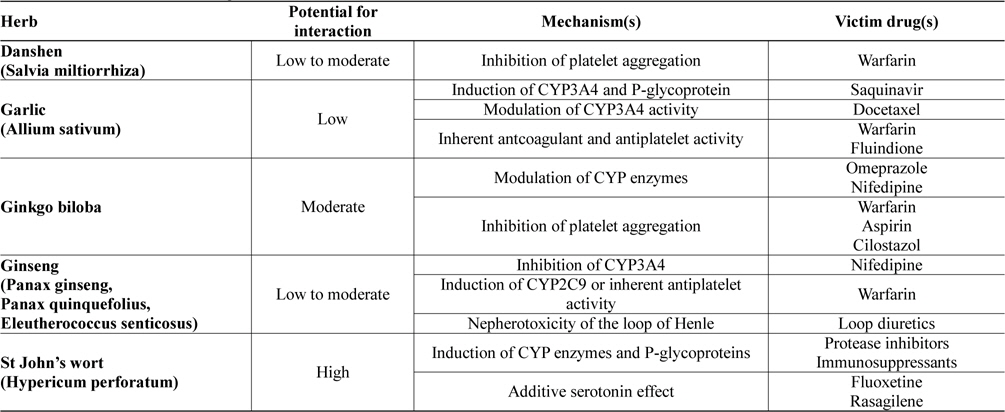
Potential drug-herb interactions have been reported in a wide variety of laboratory, animal and human studies. Furthermore, herbal remedies have been shown to interact clinically with many prescribed drugs. Table 1 lists some of the most commonly used herbs which have been reported to cause drug interactions. Discussion on how these herbal remedies may affect the pharmacokinetic and pharmacodynamic profiles of prescribed drugs will be discussed in the ensuing sections.
[Table 1.] Clinical relevance of drug-herb interactions
Clinical relevance of drug-herb interactions
Danshen, the dried root and rhizome of Salvia miltiorrhiza Bunge, is a widely used medicinal plant in China and a complementary medicine in the West. Because of its properties in improving microcirculation, causing coronary vasodilatation, inhibiting platelet adhesion and aggregation, suppressing the formation of thromboxane, and protecting against myocardial ischemia, it is widely used either alone or in combination with other herbs for cardiovascular and cerebrovascular diseases including angina pectoris, thrombosis, hyperlipidaemia, acute ischaemic stroke and hypertension (Cheng, 2007; Gao et al., 2012; Lam et al., 2006). Danshen is indexed in the 2010 Chinese Pharmacopoeia, with more than 35 formulations and concoctions containing Danshen water-extracts, ethanolic extracts or their combination, each with descriptions and details on the standards of purity, testing, dosage, precautions, storage, and the strength. Danshen products may be standardized according to major constituents which include tanshinones (dipertenequinones), salvianolic acid (a polyphenolic acid) and related danshensu compound, 3,-4-dihydroxyphenyllactic acid (Gao et al., 2012). This herb possesses low to moderate potential to interact with prescribed drugs and one important drug group affected is the anticoagulants which include warfarin.
Case reports and animal data indicate that danshen can increase the effects of warfarin resulting in bleeding. The anticoagulant effect of warfarin was reported to be increased in two patients as a result of ingestion of danshen (Izzat et al., 1998; Yu et al., 1997). In both cases, patients’ international normalized ratio (INR) was dramatically raised (to more than 5, from the target range of 2 - 3) after danshen intake. After discontinuing danshen, the patients were able to be stabilized on warfarin with INR back to desired range in the absence of danshen. Animal studies in rats demonstrate that administration of danshen aqueous extract was able to prolong prothrombin time and increase the steady state levels of both isomers of warfarin (Chan et al., 1995; Lo et al., 1992). The mechanism of this interaction is uncertain but animal and in vitro studies suggest that danshen may impair haemostasis with its antiplatelet actions and therefore the action may be additive to the anticoagulant effect of warfarin. Available evidences suggest that interaction of danshen with drugs via modulation of CYP activities seems unlikely and is of little clinical relevance. Limited in vitro and animal studies indicate that danshen had inductive effect on CYP1A2 activity but the effect appeared to be limited to ethyl extracts of danshen (Kuo et al., 2006; Ueng et al., 2004), and not the aqueous extract which is the form more commonly consumed by patients. This effect may not be clinically relevant, as clinical studies with theophylline, the substrate probe for CYP1A2, did not find a clinically relevant interaction (Qiu et al., 2008). Similarly studies on CYP2C9 and CYP3A4 (using tolbutamide and nifedipine as probes) also indicate negligible interaction with danshen (Kuo et al., 2006). Therefore there remains minimum risk of pharmacokinetic interaction between danshen and prescribed drugs via modulation of CYP activity.
Considering all the available evidences, it is possible that danshen, by its platelet inhibitory effect, may increase bleeding risk in patients stabilized on warfarin and other anticoagulants. It is therefore important for patients taking anticoagulants to be closely monitored and be advised to stop consuming danshen or use an alternative. Risk for interaction of danshen with CYP drug substrates, on the other hand, appears to be remote as negligible pharmacokinetic interactions have been reported.
Garlic (Allium sativum) a common food and flavouring agent used throughout the world, is one of the earliest known cultivated plants and is one of the most frequently used herbal supplements. It has been generally used for the treatment of hypercholesterolaemia, prevention of arteriosclerosis, and other conditions such as cancer, because it is believed to have antibacterial, antiparasitic, antilipidemic, antihypertensive, anticancer and antioxidant activities, and to improve immune function. Garlic contains a large number of biologically active constituents. These include more than 30 organic sulfur compounds derived from cysteine derivatives such as S-allyl-L-cysteine. Alliin (S-ally-L-cysteine sulfoxide) and related compounds are converted into thiosulfinates responsible for the familiar odour of fresh garlic. Allicin (diallyl thiosulfinate), one of the more highly studied components of garlic, is not present until the garlic bulb is crushed, releasing the enzyme alliinase, which converts the sulfoxides to their respective thiosulfinates. The thiosulfinates may eventually be reduced to sulfides that are responsible for unfavourable breath odour after garlic consumption (Block, 1985; Singh and Singh, 2010). Garlic has in general low potential for interaction, but clinical studies and case reports have reported the ability of this herb to interact with some important drug groups including protease inhibitors, taxane anticancers, anticoagulants and antiplatelet agents. Some of these interactions may warrant close monitoring of drug therapy to minimize adverse event or therapeutic failure.
Garlic is commonly used by HIV-infected patients to improve health and to treat some opportunistic infections, and it has the potential for interactions with anti-HIV drugs including protease inhibitors (Borrelli et al., 2007). A clinical study in healthy subjects has indicated that garlic supplementation resulted in a significant decline (as much as 54%) in plasma concentrations of saquinavir (Piscitelli et al., 2002) but the same was not observed in another study with ritonavir (Gallicano et al., 2003). The mechanism of interaction with saquinavir is uncertain, but it is thought that garlic reduced the bioavailability of saquinavir by induction of CYP enzymes, in particular, CYP3A4, as well as P-glycoprotein in the intestine (Piscitelli et al., 2002). Garlic effect on other protease inhibitors is unknown and therefore this interaction may not be generalized and apply to other antiretroviral agents. However, the magnitude of reduced saquinavir concentration as reported in Piscitelli et al. (2002) is expected to decrease antiviral effect in some patients. As such, patients taking saquinavir should avoid chronic and high-dose garlic administration. Interaction of garlic with docetaxel, another CYP3A4 substrate, has also been reported. Systemic clearance of docetaxel was reduced by 65 - 77% in ten women with metastatic breast cancer when coadministered with garlic (Cox et al., 2006). Thus, combination of the anticancer with garlic may warrant patient monitoring. Because garlic has complex cardiovascular effects, including anticoagulant and antiplatelet activity, its potential to interact with anticoagulant or antiplatelet drugs cannot be ruled out. One case report suggested that garlic can increase INR in patients previously stabilized on warfarin (Vaes and Chyka, 2000) and another reported decreased INR in patients receiving another anticoagulant fluindione (Pathak et al., 2003). These observations however were not supported by a placebocontrolled study which demonstrated the lack of effect on INR with concurrent garlic administration (Morris et al., 1995). The available data are thus not sufficient to establish the clinical importance of the interactions.
Overall, interactions of garlic with saquinavir, docetaxel, warfarin and fluindione have been reported. The causal relationship and clinical importance of these interactions have not been well established. While further information is awaited, caution is advised if garlic is taken concomitantly with the above mentioned drugs or other drugs from the same therapeutic classes.
The ginkgo is the oldest living tree species, geological records indicate this plant has been growing on earth for 150 to 200 million years (Defeudis and Drieu, 2000). Chinese monks are credited with keeping the tree in existence, as a sacred herb. It was first brought to Europe in the 1700' s and it is now among the most popular herbal remedy worldwide. Ginkgo biloba is used for the treatment of cerebral insufficiency or peripheral vascular disease, and is frequently taken for enhancement of memory function (Weinmann et al., 2010). The flavonol glycosides (including aglycone and glycosides of kaempferol, quercetin, and isorhamnetin) and terpene trilactones (such as bilobalide, ginkgolide A, ginkgolide B, ginkgolide C, and ginkgolide J) are the major constituents of G. biloba (van Beek and Montoro, 2009). Some of these constituents (ginkgolide, bilobalides and others) have antiplatelet activity and are platelet-activating factor (PAF) receptor antagonists (Bone, 2008), hence the main use of this herb in neurological disorders and circulation problems. The herb has moderate potential for drug interactions where interaction with prescribed drugs including proton pump inhibitors, calcium channel blockers and drugs affecting blood coagulation (anticoagulants and antiplatelet agents) have been reported (Shi and Klotz, 2012).
The effect of G. biloba on various CYP isoforms has been widely investigated in clinical studies. In most studies, G. biloba had no significant effect on CYP1A2, CYP2D6, CYP2E1, CYP2C9 or CYP2B6 (Greenblatt et al., 2006; Gurley et al., 2005; Lei et al., 2009; Markowitz et al., 2003). One of the studies examined the effect of the herb on the pharmacokinetics of omeprazole in Chinese subjects previously genotyped for CYP2C19. Twelve days of treatment with G. biloba induced hydroxylation of omeprazole in a CYP2C19 genotype-dependent manner. Concurrently, renal clearance of 5-hydroxyomeprazole was reduced (Yin et al., 2004). This study has demonstrated the ability of G. biloba in inducing CYP2C19 activity and the inductive effect may probably apply to other major CYP2C19 substrates in addition to omeprazole. Therefore caution should be exercised when co-administering CYP2C19 substrates (in particular omeprazole) and G. biloba. The effect of G. biloba on another isoform CYP3A4 is less consistent. In one clinical trial, G. biloba decreased the area under the plasma concentration-time curve (AUC) and maximum concentration (Cmax) of midazolam by 34% and 31%, respectively, suggesting that G. biloba could induce CYP3A (Robertson et al., 2008). Oral ingestion of the herb did not significantly affect the pharmacokinetics of nifedipine, another CYP3A4 substrate. However, in two subjects, the Cmax of nifedipine was approximately doubled. In addition, both subjects had more severe and longer-lasting headaches, dizziness or hot flushes, and a trend towards higher heart rates when G. biloba was co-administrated (Yoshioka et al., 2004). In the case of anticoagulants and antiplatelets, isolated cases of bleeding have been reported following the use of G. biloba with warfarin and aspirin (Matthews, 1998; Rossenblatt and Mindel, 1997). In another study involving another antiplatelet, cilostazol, prolonged bleeding time was observed when G. biloba was added to patients taking cilostazol even though platelet aggregation, clotting time and platelet count were unaltered (Aruna et al., 2006). In vitro evidence suggests that G. biloba inhibited platelet aggregation, but the clinical relevance of this effect is not established (Bone, 2008). Nonetheless, the combination of drugs affecting blood coagulation with G. biloba should generally be avoided due to the potential for serious bleeding complications which can be life-threatening.
In conclusion, G. biloba has been shown to modulate some CYP isoforms and platelet aggregation. G. biloba-drug interactions were also demonstrated in some clinical studies. While potential for interaction is moderate only, caution should still be exercised with combination of G. biloba and the above mentioned drugs.
For centuries ginseng has been used as a traditional herbal medicine in many Asian countries. Today ginseng products are consumed worldwide and rank among the most commonly used herbal products. Several species of ginseng are available on the market including Panax ginseng (Asian ginseng), Panax quinquefolius (American ginseng) and Eleutherococcus senticosus (Siberian ginseng). Ginseng has diverse pharmacological activities, including effects on the central nervous system, antineoplastic and immunomodulatory effects. Ginseng products are advocated for many conditions, including maintenance of general health, physical and mental well-being, treatment of fatigue, weakness and mild depression, improving immune function, and for conferring antioxidant effect (Attele et al., 1999). Its major active components are ginsenosides, a group of steroidal saponins, which exhibit most of the pharmacological actions above (Qi et al., 2011). The herb possesses low to moderate potential for drug interactions. Major groups of drugs reported to be affected include calcium channel blockers, anticoagulants and loop diuretics.
Interaction with calcium channel blockers appears to involve modulation of CYP activity. In vitro effect of ginseng on CYP isoforms have been investigated (Chang et al., 2002; Foster et al., 2002; He et al., 2006; Yu et al., 2005). Most studies however showed only weak to moderate degree of inhibition or induction, and the effects are not likely to be clinically significant. Clinical trials or case reports on ginseng-drug interactions are limited. Ginseng inhibition of CYP3A4 was statistically significant in a clinical trial (Smith et al., 2001). The magnitude of the effect however was rather modest with increase of only 29% in plasma concentration of nifedipine, the CYP3A4 substrate probe. The increase of this magnitude would be expected to produce only small changes in patient response. Potential interactions between ginseng and warfarin have been documented in case reports whereby the warfarin anticoagulant effect (measured by INR or warfarin plasma concentration) was found reduced (Janetzky et al., 1997; Rosado, 2003). Although mechanism of this interaction is not clear, modulation of CYP activity might be one of the possible reasons. Induction of CYP2C9, the most important warfarinmetabolizing isoform, has been proposed as the mechanism (Gurley et al., 2002) although further studies are needed to verify it. Another mechanism could be the antiplatelet activity of ginseng. In vitro experiments have found that the herb contains antiplatelet components that inhibit platelet aggregation and thromboxane formation (Kuo et al., 1990). This activity however was not demonstrated in a study with healthy subjects (Beckert et al., 2007). In a case report of concurrent intake of furosemide and ginseng in a patient, ginseng appeared to inhibit diuretic response to the loop diuretic where temporal relationship between ginseng ingestion and resistance to furosemide was observed (Becker et al., 1996). It was possible that ginseng may have induced nephrotoxicity involving the loop of Henle resulting in refractoriness to furosemide. The clinical importance of this effect is nevertheless not well established and more controlled studies are needed to confirm it.
In summary, the available clinical evidences suggest that the potential for ginseng-drug interactions is low to moderate only. Caution is however advised when patients are taking anticoagulants and loop diuretics together with ginseng, and patient response to drug therapy should be monitored and assessed.
St John’s wort (Hypericum perforatum), one of the oldest and best investigated herbal remedies, is the most commonly used herbal antidepressant for treatment of mild to moderate depression (Solomon et al., 2011). H. perforatum is a yellow-flowering, perennial herb indigenous to Europe that has been introduced to many temperate areas of the world and grows wild in many meadows. The common name comes from its traditional flowering and harvesting on 24 June, the birthday of John the Baptist (St John’s Day). Many clinical trials have demonstrated efficacy of the herb to be superior to placebo and comparable to standard antidepressants but with fewer side effects (Linde et al., 2008). When used as a single agent, a favourable risk/benefit ratio has made St John’s wort one of the most readily consumed dietary supplements in the world. Its popularity however has also contributed to its distinction as being one of the most problematic dietary supplements with regard to drug-herb interactions. The herb does indeed possess high potential for drug interactions, and by far, it is one of the most extensively studied herbs in relation to drug-herb interactions (Vlachojannis et al., 2011). Combination of the herb with protease inhibitors (including indinavir, ritonavir, saquinavir, darunavir and atazanavir), oral contraceptives, immunosuppressants (including cyclosporine and tacrolimus), and psychoactive drugs affecting serotonergic pathway (including rasagiline) should be avoided. If combination is unavoidable, close monitoring of drug response and dose adjustment should be instituted in patient management.
Hyperforin, an active constituent of St John’s wort, appears to be an inducer of many CYP isoforms and it may also induce P-glycoprotein (Butterweck et al., 2007). Both mechanisms lead to reduced plasma concentrations of victim drugs. Clinical studies investigating interaction between protease inhibitors and the herb showed that administration of St John’s wort resulted in decrease of AUC and Cmax of the antiretroviral agents (de Maatet al., 2001; Hafner et al., 2010; Piscitelli et al., 2000). Reduction of antiviral efficacy as well as possible resistance to these protease inhibitors may thus occur in patients. Similarly, subtherapeutic cyclosporine and tacrolimus levels have been reported in organ transplant patients ingesting St John’s wort (Hebert et al., 2004; Mai et al., 2004). Heart, kidney, liver and pancreas transplant rejection episodes, with decreased cyclosporine levels, were demonstrated after patients started St. John’s wort. In studies involving tacrolimus, the herb was able to decrease AUC of the immunosuppressant by 58%. In both cases of cyclosporine and tacrolimus, it was necessary to increase the median doses by 65% and 78% respectively to maintain therapeutic levels (Bauer et al., 2003; Mai et al., 2003). Potential pharmacodynamic interactions resulting in serotonin syndrome can occur when St John’s wort is used with other serotonergic drugs, as a result of additive serotonin effects (Bonetto et al., 2007; Dannawi, 2002). Serotonin syndrome has been reported in patients who use both St John’s wort and serotonergic drugs including fluoxetine and rasagiline. The concurrent use of these drugs with the herb should be avoided because of increased risk of the neurologic adverse effect, and where combination is unavoidable, proper lapse time should be instituted between dosing of the drugs and the herb.
Based on the discussion above, it is apparent that a large number of studies have indicated that St John’s wort can cause both pharmacokinetic and pharmacodynamic interactions. The potential for drug-herb interactions is high; therefore, patients taking prescribed drugs should be discouraged from taking herbal products containing St John's wort.
Drug-herb interactions listed in Table 1 have provided evidence that co-administration of prescribed medicines and herbal products could lead to serious adverse reactions caused by interactions. While potential for interaction remains low for many herbs, there are some which have demonstrated moderate to high potential. There is therefore a necessity for adequate pharmacovigilance to be carried out in minimizing unanticipated but often preventable drug-herb interactions. During the course of drug treatment, patients should be encouraged to tell their health care providers which herbal remedies they are taking, and the health care providers should be sufficiently informed to provide useful advice for their patients. Availability and application of this information is critical, particularly in elderly patients who are at higher risk of adverse interactions, especially as polypharmacy is often practised in this population (Klotz, 2009). Thus, increased knowledge of drug-herb interactions would enable health care providers and patients to be alert to the potential of interactions, and to improve the treatment outcomes.