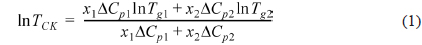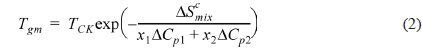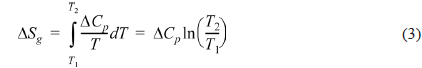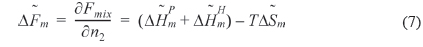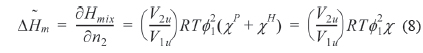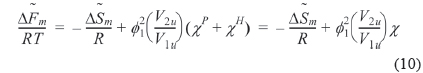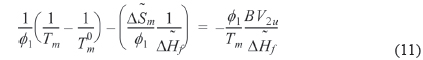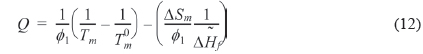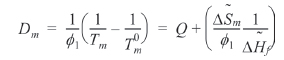Poly(vinyl alcohol) (PVA) and its blend with polyethyleneimine (PEI) have attracted great interest due to their potential application in functional membranes as well as for fibers [1-8]. The blends are also a good model system for miscible polymer blends with strong hydrogen bonding. Graphene oxide (GO) is a chemically exfoliated graphite, which is a precursor for graphene (or reduced GO) sheets [9-11]. Graphene has emerged as an ingredient for polymer nanocomposites due to its exceptional electron transport [12-14], mechanical and rheological properties [15-17], and gas barrier properties [15,18,19]. Molecular level dispersion of GO has been reported in the PVA/GO system, which may be ascribed to the hydrogen bonding interaction between the components [18,20].
Hydrogen bonding in polymer blends has been of interest to polymer scientists for several decades [21-39]. One major characteristic in such systems is the large positive deviation of glass transition temperature (
At equilibrium melting temperature, the chemical potential of the crystalline phase is equal to that of the liquid phase [44,45]. If the crystalline phase sustains the pure state, a new equilibrium condition arises where the chemical potential of the crystalline phase is equal to the reduced-chemical potential of the mixedliquid phase, which leads to the depression of equilibrium melting temperature [45]. Therefore, either the negative entropy of mixing or the positive enthalpy of mixing by strong hydrogen bonding may weaken the depression of melting temperature. Unfortunately, the melting point depressions could not be examined in the amorphous blends of PVPh and PVPy by Painter and coworkers [37]. Subsequently, Painter and coworkers [46] reported the effect of hydrogen bonding on the melting point depression in polymer blends where one component crystallizes. However, the abnormal increase of
Blends of PVA/PEI may be a good alternative model system for investigating both melting behaviors and glass transition temperatures, since PVA is a semi-crystalline polymer. PEI is a multifunctional aliphatic amine with strong cationic characteristics, whereas PVA is a water-soluble poly hydroxyl polymer. Amino groups in the PEI chain could also form strong hydrogen bonds with hydroxyl groups in the PVA chain.
In this work, we have explored the thermal properties of PVA/PEI/GO blend systems. In particular, we investigated the glass transition temperatures and the equilibrium melting temperatures. We evaluated the configurational entropy of mixing from the glass transition temperatures in the strong hydrogen bonding systems. The effect of strong hydrogen bonding on the degree of depression of equilibrium melting points was also investigated. We also examined the effect of GO on the thermal behaviors of PVA/PEI blends.
PVA was purchased from Sigma Aldrich (99 mol% hydrolyzed, Mw = 89 000-98 000 g). PEI, 50 wt% aqueous solution with the low molecular weight of 2000 including amino groups (ratio of primary/secondary/tertiary amino groups was roughly 1/2/1), was purchased from Sigma Aldrich. Flake graphite powder (19 μm nominal particle size) was supplied from Asbury Carbon. GO was synthesized from purified conventional flake graphite by modified Hummers method [9] as reported in our earlier work [17,18].
2.2. PVA/PEI/GO blend preparation
PVA solution was prepared by dissolving pure PVA powder with distilled water at 363 K for 1 h and stirring at the same time. Then, the PVA solution was cooled to room temperature and mixed with PEI aqueous solution at a designated mixing ratio by stirring for 1 h. A homogenized solution was prepared in a bubble-free state. A colloidal solution of GO was added to the PVA/PEI solution with a designated mixing ratio and the PVA/PEI/GO solution was milled for 0.5 h at 298 K. Then, the solution was cast on glass plate at 373 K and dried in a convection oven at 373 K for 3 h followed by drying in a vacuum oven at 393 K for 3 h to ensure the removal of moisture.
2.3. Characterization of PVA/PEI blends
The morphology of the blends was characterized with field emission scanning electron microscopy (FE-SEM; JEOL JSM-6700F) by observation of the cryogenically fractured surface of samples. High-resolution transmission electron microscopy (HRTEM) was performed with a Hitachi HF-2000 operated at 200 kV. Samples were applied onto a 400 mesh Cu grid with lacey carbon film and dried in air before TEM imaging. X-ray photoelectron spectroscopy (XPS) was performed in a Multilab2000 with a Mg-K X-ray source using a power source of 300 W. The atomic fractions of the different elements in the 10 nm upper layer were probed by XPS and calculated from the survey spectra. X-ray diffraction (XRD) analyses were performed directly on the hybrid samples using a Rigaku (Japan)- Ultima IV (XRD; 40 kV, 40 mA) with Cu irradiation at a scanning rate of 0.02/s in the 2
3.1. Glass transition temperatures (TgS) of PVA/PEI blends
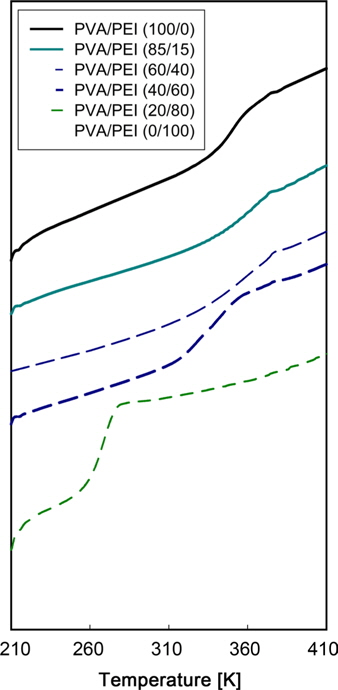
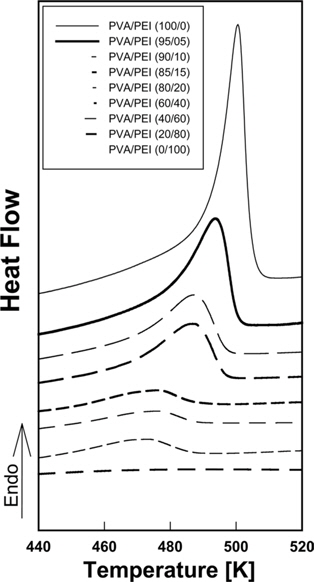
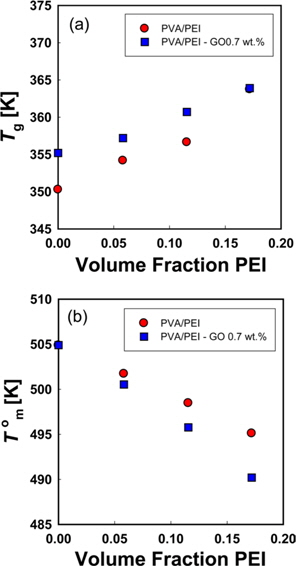
DSC thermograms for PVA/PEI blends are seen in Fig. 1. The glass transition temperatures (
where
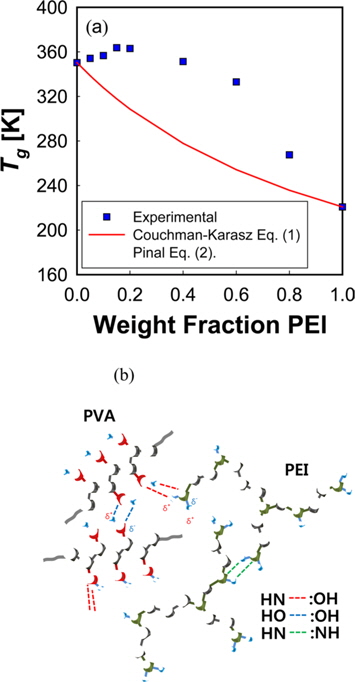
A large positive deviation from the calculated values can be observed in Fig. 2a. Specifically, the
According to Gibbs and DiMarzio [47], the glass transition temperature may be defined as the point where the configurational entropy becomes zero when a polymer in melt state is cooled down to a glassy state. OH--N bonding may be formed in PVA/PEI blends, as seen in Fig. 2b, which may be stronger than OH--O bonding in PVA itself or than NH--N bonding in PEI. So, the strengthened hydrogen bonding produced by mixing may induce a decrease of the configurational entropy in the blend at melt state. This may lead to the abnormal increase of
where
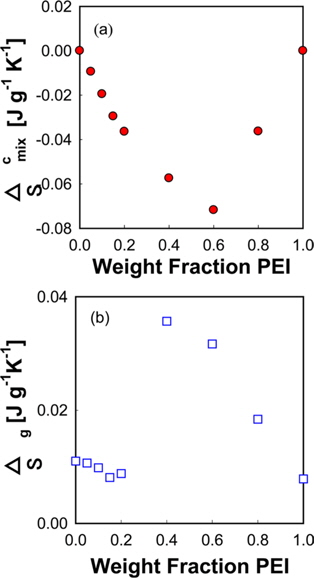
The entropy change of the PVA/PEI blends in the glass transition region can be obtained by Fig. 3b according to the definition of entropy by Clausius [44].
Δ
3.2. Melting behaviors of PVA/PEI blends
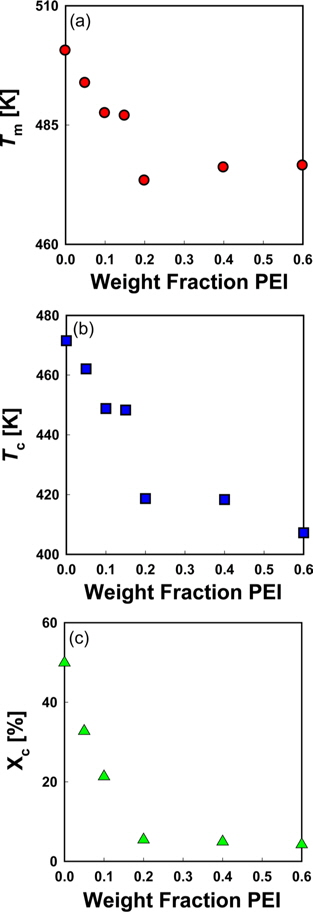
DSC thermograms for melting peaks of PVA in PVA/PEI blends are presented in Fig. 4. The non-isothermal melting temperatures (
where
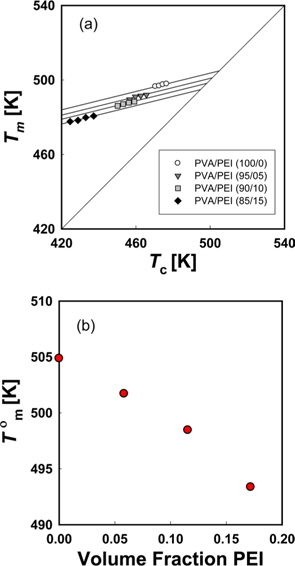
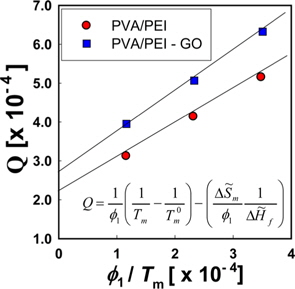
Fig. 6a is a Hoffman-Week’s plot [48] for the estimation of equilibrium melting temperature (
The left hand side of Eq. (5) is the molar free energy of fusion and the right hand side is the partial molar free energy of mixing with respect to the crystalline component (). In the case of a hydrogen-bonding system, the free energy of mixing can be divided into two terms, as in Eq. (6) [37].
where and is the partial molar free energy of mixing contributed by the hydrogen bonding force and by all other forces, respectively. Usually, is given by the Flory-Huggins equation. Eq. (6) can be re-written as Eq. (7).
where
where
By setting equal to the molar free energy of fusion and inserting Eq. (9) into Eq. (10), the following expressions can be obtained
where is athermal in the liquid state. We refer to the left hand side of Eq. (11) as
We can refer the first term in Eq. (12) to be the degree of the depression of melting point (
The
where
Since the entropy of mixing of the blends of PEI and PVA has a negative sign, it can be supposed that the
3.3. Effect of GO on the thermal behaviors of PVA/PEI blends
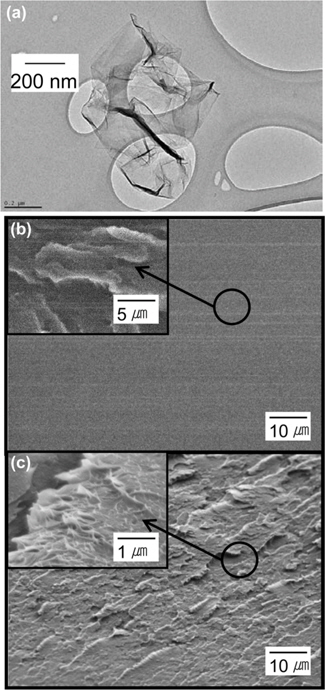
GO was prepared from purified conventional flake graphite (19 μm nominal particle size, Asbury Carbon) by the modified Hummers method [9,17,18]. The TEM image of the reduced GO sheets suspended on a carbon grid are presented in Fig. 7a, showing the typical image of graphene with electron transparency. We investigated the degree of oxidation of the GO by XPS. The carbon to oxygen atomic ratio of GO was determined to be 1.8 from the XPS spectra. We dispersed 0.7 wt% GO into the blends of PVA and PEI. The SEM images of the surface of the blend films are presented in Fig. 7b and c. The PVA/PEI blends are thermodynamically miscible to form a single phase, therefore, no appreciable domain is observed in Fig. 7b. A rough layer-like morphology is observed on the surface of the PVA/ PEI/GO blends in Fig. 7c. The XRD patterns of the PVA/PEI/ GO blends show no GO characteristic peak at 2
The
The