Herbicides are widely used in rice paddies, golf courses and other types of fields. The acetanilide herbicide butachlor and the sulfonylurea herbicide pyrazosulfuron-ethyl are pre-emergence herbicide to control weeds of transplanted rice paddy fields (Chen and Chen,1979; Zheng
All analytical grade solvents (acetonitrile, acetone, acetic acid, methanol, phosphoric acid) were purchased from Wako Chemicals (2011, Tokyo, Japan). Deionized water used for sample preparation and mobile phase was produced with a Milli-Q water purification system (Millipore, Billerica, MA, USA). Analytical standard grade butachlor (98%) and pyrazosulfuronethyl (98%) were also purchased from Wako Chemicals. The primary individual stock solutions (50 mg/L) of each analytical standard were prepared in 100 mL volumetric flask with pure acetonitrile. These stock standard solutions were diluted with acetonitrile to obtain a final concentration of 10 mg/L and kept in fridge at 4°C. The 10 mg/L standard stock solutions were appropriately diluted in acetonitrile:water (20:80, v/v) to prepare working standards 0.5 mg/L and 0.01 mg/L of butachlor and pyrazosulfuron-ethyl, respectively.
The paddy water was collected from the experimental paddy field at FM Honmachi of Tokyo University of Agriculture and Technology (TUAT), Japan. Paddy water samples were filtered through 1.2 μm glass fiber filters and sterilized by autoclave at 120°C for 20 minutes for the experiments. These solutions were divided into 24 sample bottles of 250 mL and the standard solution was spiked in water sample bottles. The 12 bottles for treatment were covered by quart glass plates, which allow about 90% of UV radiation (280-2000 nm) to pass through, to minimize the UV attenuation. The other 12 bottles for the control were covered by glass lids and wrapped with aluminum foils to prevent the sunlight. The concentration of butachlor and pyrazosulfuron-ethyl in water sample bottles at initial, 1, 3, 7, 14 and 21 days was monitored under ambient conditions with and without sunlight from May 5 to May 26 in 2011 at the university farm of TUAT. The solar radiation was monitored by pyrheliometer placed next experimental field. The water temperature was also monitored by temperature sensors placed in the water samples.
Prior to the extraction, water samples were filtered by 1.2 μm glass fiber filter paper (GC/C-Whatman). The water samples of pyrazosulfuron-ethyl were adjusted to pH 2.5 by acid phosphoric and then both sample of butachlor and pyrazosulfuron-ethyl were extracted by solid phase extraction method using the Superclean ENVI-18 as previous study by Ok
Monitored average temperatures of tested paddy water sample in the treatment and control in this experiment were 20.9 ± 7.8 and 19.5 ± 5.8°C, respectively. The temperature at noon of the treatment seems to be higher than the control due to the heat supplied by the sunlight. However, over the course of experiment, the difference of mean temperature between control and treatment in this experiment was insignificant. The cumulative ultraviolet B(UVB) radiation energy of the natural sunlight during 21 days after herbicide application was 321.4 kJ/m2 in this experiment. The pH values in paddy water was 7.4 ± 0.1 for treatment and 7.6 ± 0.2 for control, which were in similar range to monitored data in micro paddy lysimeter experiment (Ok
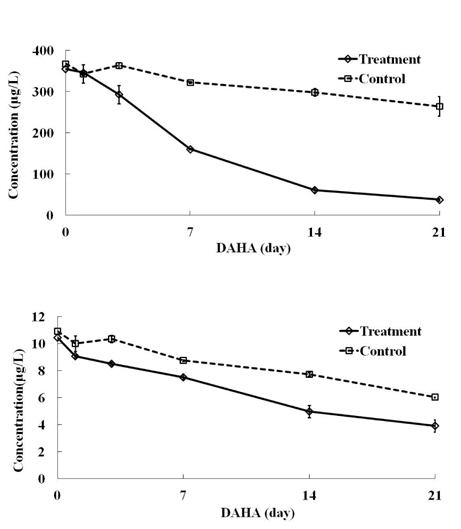
The concentrations of butachlor and pyrazosulfuronethyl during consecutive 21 days after herbicide application under natural sunlight are shown in Fig. 1. The average concentration of butachlor at initial day was 355.3 μg/L for treatment and 367.9 μg/L for control, and the corresponding values for pyrazosulfuronethyl was 10.5 μg/L for treatment and 10.9 μg/L for control. The average concentration of both herbicides was similar as the concentration range in the actual paddy field and micro paddy lysimeter experiment (Ok
[Fig. 1.] Concentrations of butachlor and pyrazosulfuronethyl in paddy water under natural sunlight.
The half-life (DT50) during 21 days after herbicide application was determined using first-order kinetics. The DT50 was obtained as the slope of natural logarithm of pesticide concentrations (μg/L), ln(c), versus the exposure period (hour, hr). The results are summarized in Table 1. The estimated DT50 of butachlor in paddy water during 21 days after herbicide application were 6.0 days for treatment and 46.5 days for control, respectively. The butachlor were very sensitive to sunlight that the half-life of butachlor was about 5.4 hr in aqueous solution (Chen and Chen, 1979) and 58.2 ± 4.5 hr in filtered river water under sunlight (Lin
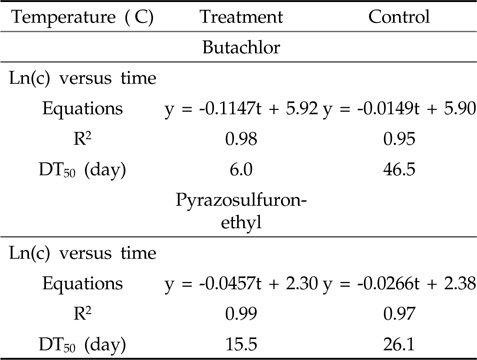
The half-life(DT50) (day) of butachlor and pyrazosulfuron-ethyl in paddy water under natural sunlight
On the other hand, the estimated DT50 of pyrazosulfuron-ethyl in paddy water during 21 days after herbicide application were 15.5 days for treatment and 26.1 days for control. The sulfonylurea herbicides class indicated that photodegradation is an alternative pathway to chemical hydrolysis (Samanta
The degradation processes of herbicides, butachlor and pyrazosulfuron-ethyl, in rice paddy water under natural sunlight were investigated. The concentration of butachlor in paddy water decreased quickly under ambient conditions with natural sunlight. The degradation of butachlor in paddy water was enhanced by the natural sunlight. However, the degradation of pyrazosulfuron-ethyl was insignificant under natural sunlight.