Recently, the generation of white light through a combination of an ultraviolet or blue-emitting chip and phosphors as a solidstate lighting source has attracted considerable interest [1,2]. These phosphors and chip-based light-emitting diodes (LEDs) have several advantages over conventional incandescent and fluorescent lamps, in terms of power efficiency, long lifetime, absence of pollution, and design flexibility [2,3]. White lightemitting diodes (W-LEDs) have been the subject of increasing interest, due to their advantages of low energy consumption, long life span, and lack of pollutants such as Hg, and to their potential applications in indicators, backlights, automobile headlights and general illumination [4]. White light-emitting diodes (LEDs) are in high demand in solid-state lighting (SSL) technology, because of their most challenging application as a replacement for conventional incandescent and fluorescent lamps. Therefore, they are considered the next generation of solid-state lighting. Without doubt, SSL is a pivotal emerging technology that promises to alter lighting in the future. With the development of materials science and technology, soft-chemical synthesis methods such as sol-gel [5], co-precipitation [6] and hydrothermal synthesis [7,8] have been successfully applied to synthesize phosphatewhite LED phosphors. All of these methods use liquid components, which can be accurately controlled, and thoroughly mixed. Combustion synthesis is characterized by more complete reaction, increased reaction efficiency, fast reaction, and convenience.
The aim of this work is to investigate the effect of CaxSr2-x and activator on the structural and luminescent properties of green-emitting CaxSr2-xSiO4:Eu2+ nano phosphor. For small spherical particles with smooth and round surfaces, CaxSr2-xSiO4:Eu2+ phosphors were synthesized. Using urea as fuel, and ammonium nitrate as oxidizer, CaxSr2-xSiO4:Eu2+ was successfully synthesized, using a combustion method. The influence of X(CaxSr2-x) content on the crystalline structure of the produced powders of CaxSr2-xSiO4:Eu2+ phosphors was investigated. The results of characterization showed that the phosphors particles are nanosize. The phosphors exhibit a green emission spectrum for near uv excitation. The material has application as a fluorescent material for ultraviolet light-emitting diodes (UVLEDs).
In this work, we synthesize a CaxSr2-xSiO4:Eu2+ nano phosphor by a combustion method. Also, we investigate the effect of CaxSr2-xSiO4:Euy on the structural transformation and luminescent properties of green-emitting CaxSr2-xSiO4:Eu2+ nano phosphor.
In this study, CaSrSiO4:Eu2+ phosphors were prepared using a combustion method. Ca(NO3)2 (99.997%, Aldrich), Sr(NO3)2 (99.995%, Aldrich), SiO2 (99.9%, Aldrich), and Eu2O3 (99.999%, Aldrich) were used as the starting materials. The CaSrSiO4 phosphors were doped by Eu2+, with the molecular formula of CaxSr2-xSiO4:Euy 2+. Ca(NO3)2, Sr(NO3)2, SiO2 and Eu2O3 were mixed together by mol ratio, and then distilled water was added. Urea was used as fuel, and ammonium nitrate served as the oxidizer. The parameters were measured, and are shown in Table 1. A flowchart of the preparation of the phosphor powders is shown in Fig. 1. The urea and ammonium nitrate solution was heated to 80℃, and continuously stirred, using a magnetic bar. The metal solution was dropped into the fuel, and the heating was continued for 30 minutes at 80℃. The solution was then transferred to a pre-heated furnace set to 500℃. After heating, different samples of the mixture were sintered in a furnace for 3 hours, at 600℃~1,400℃. Crystalline development of the resulting samples was checked by X-ray diffraction (XRD, model D/MAX-2200), using CuKα-radiation in the range of 2 θ = 20~80°. Measurement of the photoluminescence (PL) spectra was carried out by 150 W Xe lamp (spectrofluorometer, FP-6200, JASCO). The morphology and size of the prepared particles were investigated by fieldemission scanning electron microscopy (FE-SEM, model S-4700, HITACHI).
[Table 1.] Mol ratios of the CaSrSiO4:Eu2+ used by the combustion method at various temperatures

Mol ratios of the CaSrSiO4:Eu2+ used by the combustion method at various temperatures
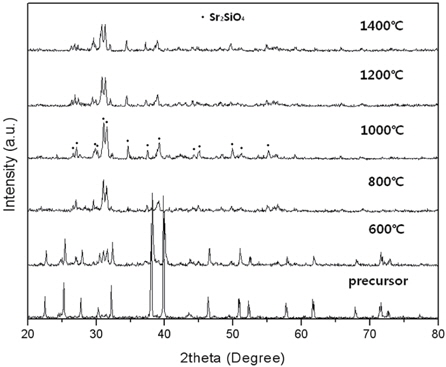
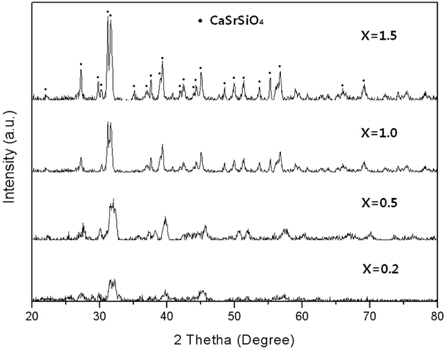
Figure 2 shows XRD patterns of the Sr2SiO4:Eu2+(X=0) sintered at different temperatures. At the different temperatures, the XRD patterns show pure Sr2SiO4 phase. In this structure, Sr2+ ions locate at two kinds of nonequivalent lattice sites, and their coordination numbers are nine and ten, respectively. When the Eu is introduced into the Sr2SiO4 structure, it takes the place of the Sr2+. In the case of precursor, not only the Sr2SiO4 phase was observed, but also impurities peaks, due to material not having been synthesized. At 1,000℃, the diffraction peaks became sharper and stronger. The Sr2SiO4 phase crystallized with results that are in good agreement. However, at 1,200℃, the Sr2SiO4 peaks were markedly weakened. Figure 3 shows XRD patterns of the CaxSr2-xSiO4:Eu2+ phosphors (x = 0.2, 0.5, 1.0, and 1.5) produced by the combustion method at 1,000℃. The structure of Ca2SiO4 is monoclinic, which is different from that of Sr2SiO4 (orthorhombic structure). Therefore, substitution of the Ca sites with Sr2+ ions might cause a monoclinic to orthorhombic structural transformation [9]. According to JCPDS card 72- 2260, pure CaSrSiO4 has a orthorhombic crystal structure with Pna21(33) space group, and lattice parameters of
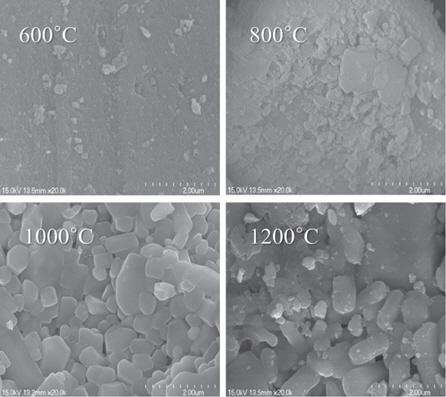
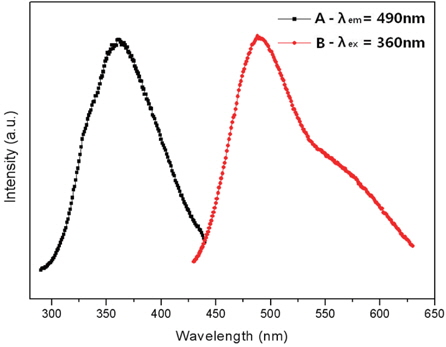
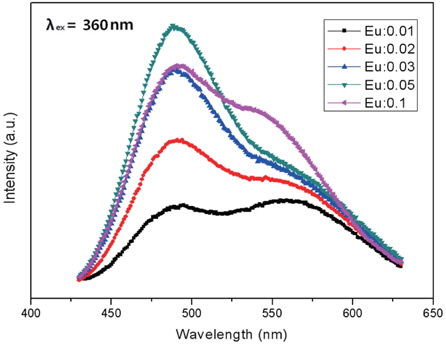
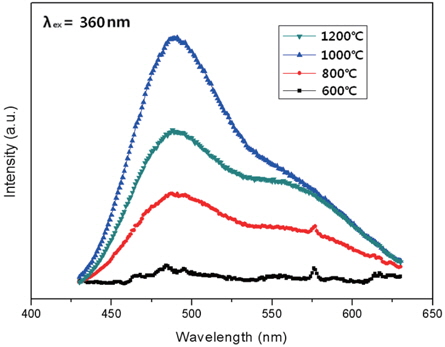
In the present research, the CaxSr2-xSiO4:Eu2+ phosphors with different concentrations of Eu2+ were prepared by a combustion method. The particles were found to be small and spherical with round surfaces. With increased sintering temperature, the phosphor particles became spherical, which occurs because the particles condense at higher temperatures. We determined that the characteristics of the phosphor powders were improved by increasing the sintering temperature. The CaxSr2-xSiO4:Eu2+ emission spectrum for 360 nm excitation showed a single band, with a peak at 490 nm, which is a green emission. The luminescent properties of the CaxSr2-xSiO4:Eu2+ phosphor were optimized by changing the Eu2+ content. As a result, the highest luminous intensity was at 1,000℃, which was obtained when the Eu2+ content (y) was 0.05. Increase of the Eu2+ concentration played a key role in enhancing the luminous intensity. In conclusion, these optimized phosphors are expected to have potential application in ultraviolet light-emitting diodes (UV-LEDs) as fluorescent material.