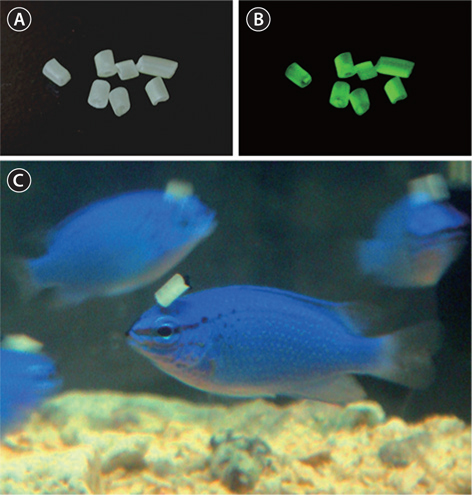
Seasonal changes in day length are critical factors affecting the initial cueing, timing, and subsequent synchronization of gonadal development in various fish species (Bromage et al., 2001; Migaud et al., 2010). Reproductive strategies involving day length are likely to be diverse among species. For example, a steady increase in day length stimulates gonadal development in the black seabass
Artificial stimulation or suppression of gonadal development by manipulating photoperiodic conditions offers potential industrial advantages for efficiently breeding fish with high commercial value at a desired time of year and for reducing the excessive use of drugs and hormones for maturation. Previous studies have used incandescent, fluorescent, metal-halide, and tungsten-halogen lamps as standard light sources and have successfully controlled the gonadal development of various fish species (Bromage et al., 2001). In terms of long-operating life and low-energy consumption, light-emitting diodes (LEDs) have recently been used as a new light source to stimulate gonadal development (Bapary et al., 2011; Leclercq et al., 2011) and growth (Yamanome et al., 2009). However, waterproofing and barotolerance issues limit the usage of this new light technology in aquaculture.
Recently, long-afterglow phosphorescent pigments (LumiNova) have been used as a light source, and light emitted by LumiNova sheets was able to prolong the reproductive season of the sapphire devil
>
Fish and experimental design
Adult sapphire devils of 3.5 ± 0.4 cm mean body length and 1.3 ± 0.6 g mean body mass were collected from coral reef lagoons (26˚25ʹ74.7ʺ N, 127˚68ʹ91.3ʺ E) in Urasoe, Okinawa, Japan, using a small round haul net during daytime low tide. Fish were transferred to and reared in aquaria equipped with filtering and aeration systems under short-day conditions (10224h photophase and 14 h scotophase; LD 10:14, light on at 08:00 and off at 18:00) and a water temperature of 26°C. A fluorescent bulb (14 W) was used as a light source, and the light intensity at the water surface was 3.6 W/m2. Fish were fed daily at 10:00 with commercial pellets (EP1; Nisshin-Marubeni, Tokyo, Japan).
LumiNova (G-300M; Nemoto Lumi-Materials, Tokyo, Japan), epoxy resin (craft resin) and hardener (Nisshin Resin KK, Yokohama, Japan) were mixed at a ratio (w/v) of 2:1:1. The mixture was poured into a silicone tube (internal diameter = 2 mm, external diameter = 3 mm) and left for 6 h at room temperature. After drying and hardening, the silicone tube containing LumiNova was cut into approximately 2-mm sections (Fig. 1A and 1B), which were kept at room temperature until use. Control pellets were prepared by mixing only the epoxy-resin and its hardener at a ratio of 2:1.
The experiment was conducted for 1 month beginning in December 2011, during the nonbreeding season of the damselfish and when their ovaries are exclusively occupied by immature oocytes (Bapary et al., 2012). After fish (
All experiments were conducted in compliance with the guidelines of the Animal Care and Use Committee of the University of the Ryukyus and the regulations for the care and use of laboratory animals in Japan.
A portion of the fixed ovary was dehydrated in an ethanol series, permutated with xylene, and then embedded in paraffin (Nacalai Tesque Inc., Kyoto, Japan). Serial sections (7 μm) were prepared and stained using Mayer’s hematoxylin–eosin for microscopic observations. Oocyte development was classified into six stages: the perinucleolus stage, oil droplet stage, yolk vesicle stage, primary yolk stage, secondary yolk stage, and tertiary yolk stage (Bapary et al., 2009).
The GSI results were expressed as the mean ± standard error of the mean (SEM). A two-way analysis of variance (ANOVA) followed by a Tukey–Kramer test was used for comparing the mean GSI among fish groups (
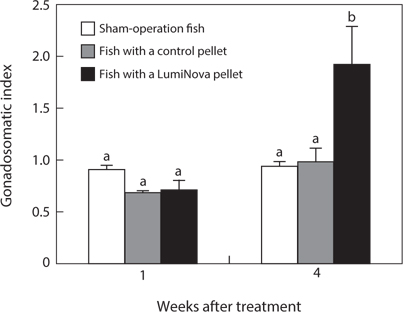
No fish died during the experimental period. The GSI of the initial control was 0.64 ± 0.12. The GSI of the treatment group increased to 0.70 ± 0.10 after 1 week and to 1.88 ± 0.37 after 4 weeks. The GSIs of the control pellet group and shamoperation group did not change over time and were 0.98 ± 0.11 and 0.92 ± 0.07 after 4 weeks, respectively. After 4 weeks, the GSI of the treatment group was significantly higher than that of the other two groups (Fig. 2).
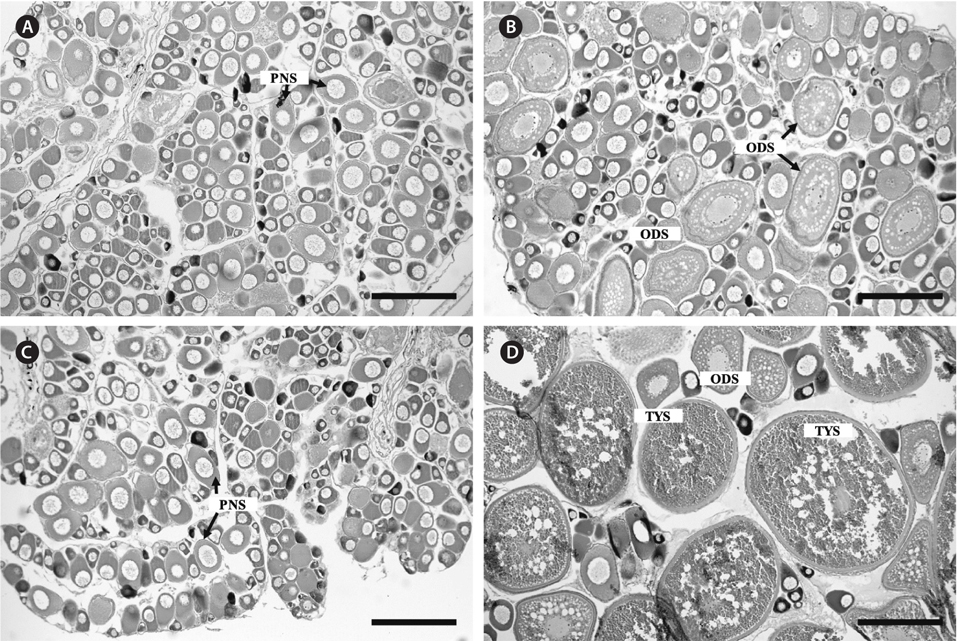
Ovaries of the initial control contained immature oocytes at the perinucleolus stage. Similar ovarian features were confirmed in the control pellet and sham-operation groups at 1 week after the start of the experiment (Fig. 3A). In contrast, ovaries of the treatment group had oocytes at the perinucleolus and oil droplet stages after 1 week (Fig. 3B). After 4 weeks, ovaries of the control pellet and sham-operation groups were occupied only by oocytes at the perinucleolus stage (Fig. 3C), whereas those of the treatment group contained fully vitellogenic oocytes at the tertiary yolk stage (Fig. 3D).
The present study clearly demonstrated that light emitted by long-afterglow phosphorescent pigments (LumiNova) stimulated ovarian development in the sapphire devil during the nonbreeding season. No ovarian development was induced during the experimental period in either control or sham-operation fish. Similar results have been obtained using the same species: fish in aquaria covered with LumiNova sheets continued active oocyte growth and repeated spawnings even after the reproductive season of naturally reared fish had terminated (Bapary et al., 2012). Thus, LumiNova emits wavelengths of light that can be perceived and utilized by the fish.
Histological observations have indicated that ovarian development of the sapphire devil in Okinawan water initiates in March and peaks in May, when both the photoperiod and water temperature are increasing (Bapary et al., 2009). When females were reared at 25°C under experimental conditions of LD 10:14, LD 12:12, and LD 14:10 using fluorescent bulbs as a light source, ovaries with fully vitellogenic oocytes were only observed under long-day conditions (Bapary et al., 2009). In addition, exposing female sapphire devils to longaquarium day conditions (LD 14:10) with red (627 nm) and green (530 nm) LED lights induced ovarian development, whereas exposure to blue (455 nm) and white LED lights did not (Bapary and Takemura, 2010). These previous reports clearly suggest that the sapphire devil perceives long-day conditions from LED lights and begins ovarian development during the natural nonbreeding season. In addition, mid- to long-wavelength lights are preferable for initiating ovarian development in this species. Because LumiNova emits green light (530 nm) in the dark after energy absorption and emits light for several hours thereafter (NEMOTO, 2000), fish are likely exposed to the desired wavelengths of light after lights are turned off. Because the photoperiodic conditions of LD 12:12 were used in the present study, the additional emission of light by LumiNova produced long-day conditions, which stimulated gonadal development.
Because the pellet was placed on the calvaria of each individual, the majority of light emitted by LumiNova likely stimulated the extraretinal photoreceptors. For example, Masuda et al. (2005) demonstrated that following pinealectomy and ophthalmectomy, gonadal development was induced under short-day conditions in the ayu sweetfish