Seaweeds are rich in vitamins, minerals, proteins, polyphenols, and polysaccharides. Therefore, several species of seaweeds are used in cosmetics, food, and pharmaceuticals. Many studies have shown that seaweeds have diverse bioactivities such as antioxidant, anticoagulant, anti-inflammation, antibacterial, and anticancer activities (Heo et al. 2003, Shin et al. 2006,
Inflammation is a normal host defense response by the organism to remove the tissue injury caused by pathogens, damaged cells, or irritants, and to initiate the healing process (Dewanjee et al. 2013). The inflammatory process is mediated by inflammatory cells, such as macrophages, neutrophils, eosinophils, and mononuclear phagocytes. Macrophages play an important role in a variety of disease processes, and are involved in the pathogenesis of autoimmune diseases, infection, and inflammatory disorders (Choi et al. 2012). And macrophages secrete inflammatory mediators such as reactive oxygen species (ROS), nitric oxide (NO), and prostaglandin mediators, which are generated by activated inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and pro-inflammatory cytokines (tumor necrosis factor-α [TNF-α], interleukin [IL]-1β, IL-6) in response to an activating stimulus, such as, the bacterial endotoxin, lipopolysaccharide (LPS) (Kim et al. 2013, Ma et al. 2013).
The vertebrate zebrafish (
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin and trypsin-EDTA were purchased from Gibco/BRL (Burlington, ON, Canada). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), 2,7-dichlorofluorescein diacetate (DCF-DA), diaminofluorophore 4-amino-5-methylamino-2′7′-difluorofluorescein diacetate (DAF-FM DA), acridine orange were purchased from Sigma (St. Louis, MO, USA). High-performance liquid chromatography (HPLC) grade solvents were purchased from Burdick & Jackson (Muskegon, MI, USA). The other chemicals and reagents used were of analytical grade.
EPB was kindly provided by Taerim Co., Ltd. (Jeju, Korea) and then was freeze-dried. The dried EPB powder (5 g) was suspended in 500 mL of 80% ethanol and mechanically stirred for 24 h at room temperature. The solution was filtered and the filtrate was concentrated under reduced pressure to give the oily extract. After the extract was suspended in 200 mL of distilled water, ethyl acetate fraction was obtained with 0.06 g as a yield and the obtained ethyl acetate fraction was used for the further experiments. Total polyphenol content was quantified by Folin-Ciocalteu methods.
HPLC and HPLC-DAD-ESI/MS analyses were performed using the protocol described by Lee et al. (2014). For processing HPLC, the mobile phase comprised of acetonitirile-water in gradient mode as follows: acetonitrile with 0.1% formic acid-water with 0.1% formic acid (0-40 min: 10 : 90 → 40 : 60, v/v; 40-50 min: 50 : 50, v/v; 50-60 min: 100 : 0, v/v). The flow rate was 0.2 mL min-1 and the UV absorbance was detected at 290 nm. HPLC-DAD-ESI / MS analyses were carried out using a Hewlett-Packard 1100 series HPLC system equipped with an autosampler, a column oven, a binary pump, a DAD detector, and a degasser (Hewlett-Packard, Waldbronn, Germany) coupled to a Finningan electrospray source and was capable of analyzing ions up to m/z 2000. MS was controlled by Xcalibur software (Finnigan MAT, San Jose, CA, USA).
Murine macrophage cell line RAW264.7 was obtained from the Korean Cell Line Bank (KCLB). The cells were maintained at 37℃ in humidified atmosphere of 5% CO2 in DMEM medium supplemented with 10% FBS and penicillin/streptomycin (100 U mL-1 and 100 μg mL-1, respectively; Gibco Inc., Grand Island, NY, USA). Exponential phase cells were used throughout the experiments.
>
Effects of EPB and PRF on LPS-induced NO production and cytotoxicity
To evaluate the effect of EPB and PRF on NO production and cytotoxicity, RAW264.7 cells were stimulated with LPS (1 μg mL-1). NO production was detected using the protocol described by Samaroakoon et al. (2013). Furthermore, cytotoxicity was assessed using the protocol described by Kim et al. (2013).
>
Western blot analysis of iNOS and COX-2 in RAW264.7 macrophages
Western blot analysis was carried out using the protocol described by Kang et al. (2011). iNOS and COX-2 expression were used specific primary rabbit polyclonal anti-rabbit iNOS Ab (1 : 1,000, Cell Signaling Technology, Danvers, MA, USA), or rabbit polyclonal anti-rabbit COX-2 Ab (1 : 1,000, Cell Signaling Technology) and goat antirabbit IgG HRP conjugated secondary antibody (1 : 3,000) in TBST.
>
Origin and maintenance of zebrafish
Adult zebrafish were obtained from a commercial dealer (Green Fish, Seoul, Korea; Stock obtained from Singapore fish dealer) and 10 fish were kept in a 3-L acrylic tank under the following conditions: 28.5℃, with a 14 : 10 h light : dark cycle. Fish were fed three times a day, 6 days a week, with Tetramin flake food supplemented with live brine shrimps (
>
Preparation of inflammation-induced zebrafish model by LPS-treatment and application of PRF
From approximately 7-9 h post-fertilization (7-9 hpf), embryos (groups = 15 embryos) were transferred to individual wells of a 24-well plate and maintained in embryo medium containing 1 mL of PRF (25, 50, and 100 μg mL-1) for 1 h. Then embryos were treated with 10 μg mL-1 LPS or co-treated with LPS and PRF for up to 24 hpf.
Generation of ROS and NO in zebrafish embryos was analyzed by using the method described by Lee et al. (2013
The data are expressed as the mean ± standard error (SE), and one-way ANOVA test (using SPSS ver. 12 statistical software; SPSS Inc., Chicago, IL, USA) was used to compare the mean values of each treatment. Values with different alphabets are significantly different at p < 0.05 as analyzed by Duncan’s multiple range test.
>
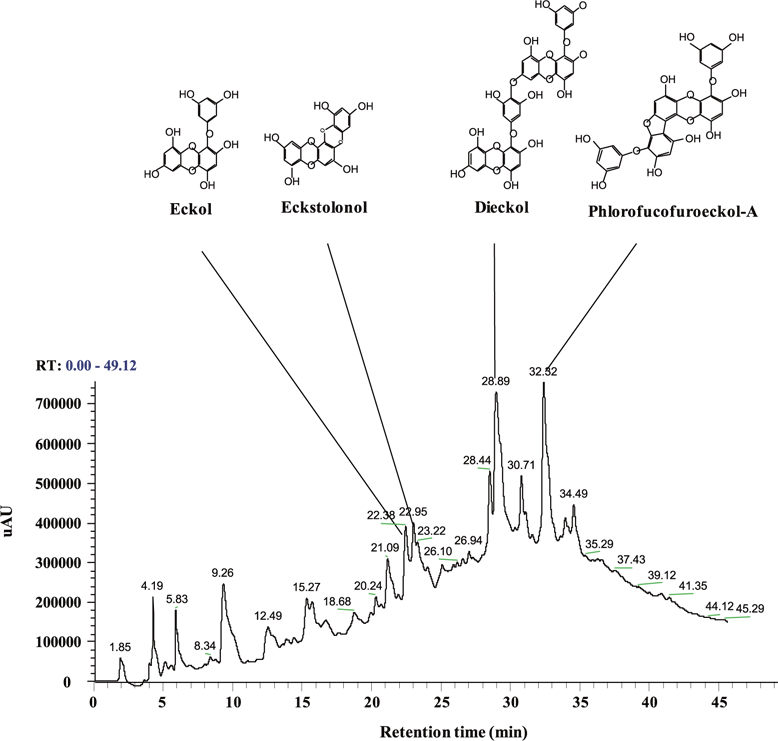
Polyphenols of the ethyl acetate fraction from EPB
Total polyphenol content of the ethyl acetate fraction from EPB was 55.81 ± 4.15%. Therefore, this ethyl acetate fraction was named the PRF. The PRF was analyzed using HPLC and HPLC-DAD-ESI / MS analyses to identify algal polyphenols (Fig. 1). The four polyphenol compounds, namely eckol, eckstolonol, dieckol, and phlorofucofuroeckol-A were confirmed by comparing their HPLCDAD-ESI / MS analyses. Thus, this ethyl acetate fraction was used in further experiments.
>
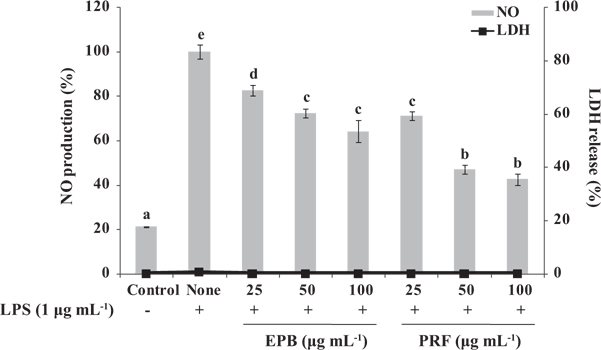
Inhibition of NO production by EPB and PRF on LPS-induced RAW264.7 cells
To examine the potential anti-inflammatory activities of EPB or PRF in LPS-induced RAW264.7 macrophages, we investigated the inhibitory effect of EPB or PRF on NO production. Cells were treated with or without EPB or PRF (25, 50, and 100 μg mL-1) for 1 h, and then treated with LPS (1 μg mL-1) for 16 h. As shown in Fig. 2, the level of NO production is significantly increased in the LPS-treated cells compared with the untreated cells. However, the NO production was significantly reduced in the cells pretreated with EPB or PRF. In particular, PRF suppressed NO production more than EPB. In addition, as confirmed by the lactate dehydrogenase (LDH) activity using an LDH cytotoxicity detection kit (Promega, Madison, WI, USA), EPB or PRF both showed non-cytotoxic effect on RAW264.7 cells at the tested concentrations (Fig. 2). Thus, PRF may be a used as a potential agent for suppressing NO production without affecting cell viability.
>
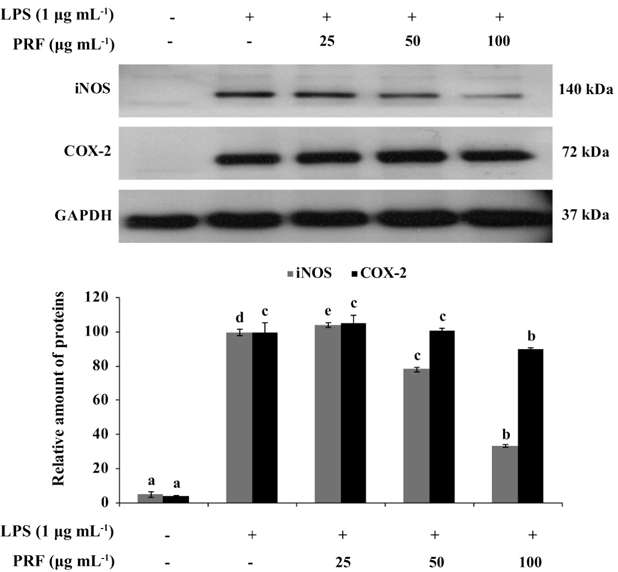
Inhibition of iNOS and COX-2 protein expression by PRF on LPS-induced RAW264.7 cells
iNOS and COX-2 are major inflammatory mediators. To determine the mechanism by which PRF reduces LPS-induced NO production, we investigated the ability of PRF (25, 50, and 100 μg mL-1) to influence the LPS-induced production of iNOS and COX-2. Fig. 3 shows the effect of PRF on iNOS and COX-2 protein expression in RAW264.7 cells by western blot analysis. The iNOS and COX-2 protein expressions were significantly increased in LPS-treated macrophages (1 μg mL-1). However, PRF significantly suppressed the protein expression of iNOS in a concentration-dependent manner and slightly inhibited COX-2 expression at 100 μg mL-1.
>
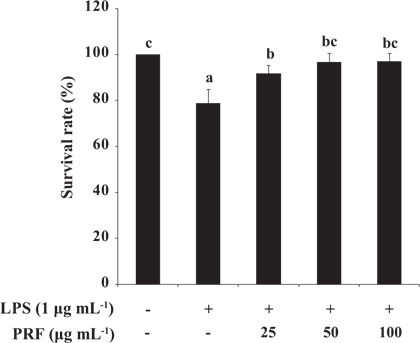
Toxicity of PRF and LPS in zebrafish embryos
To assess the toxicity of LPS (10 μg mL-1) with or without PRF, we determined the survival rates of zebrafish embryos. The survival rates of the positive control group (treated with LPS) were 78.75%, and then the survival rates of the PRF-treated embryos were significantly increased to 91.77, 96.67, and 96.88% at concentrations of 25, 50, and 100 μg mL-1, respectively (Fig. 4).
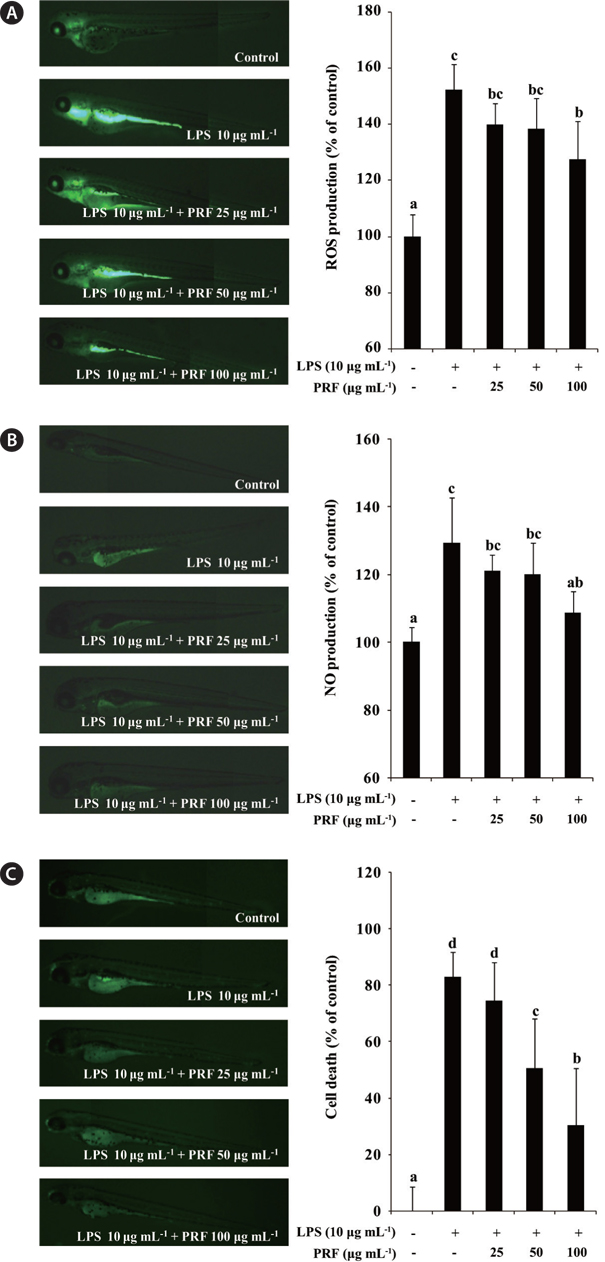
Production of intracellular ROS can be detected using the oxidation sensitive fluorescent dye, DCF-DA, emits fluorescece upon interaction with ROS (Handa et al. 2006). In the present study, therefore, the production of ROS in the LPS-induced inflammatory zebrafish model was analyzed using DCF-DA. Fig. 5A illustrates the ROS levels in zebrafish with or without LPS and / or PRF. These data show that, the ROS level in LPS-stimulated zebrafish increased to 152.22% compared with the negative control group. However, the ROS productions in zebrafish treated with different concentrations of PRF (25, 50, and 100 μg mL-1) were reduced, and a significant reduction was observed at 100 μg mL-1. We assessed the inhibitory activity of PRF on LPS-stimulated NO production in zebrafish using a fluorescent probe dye, DAF-FM DA. Transformation of DAF-FM DA by NO in the presence of dioxygen generates highly fluorescent triazole derivatives (Itoh et al. 2000, Bolck et al. 2014). The effect of PRF on LPS-stimulated NO production is shown in Fig. 5B. The level of NO production in the positive group (only LPS treated) reached to 129.39% of that observed in controls. However, zebrafish treated with 100 μg mL-1 PRF had significantly reduced NO levels.
Acridine orange is a nucleic acid selective methachromatic dye that interacts with DNA and RNA by intercalation or electrostatic attraction. Acridine orange stains cells with disturbed plasma membrane permeability, and can be used to identify necrotic or very late apoptotic cells. Fig. 5C shows that there is a high level of cell death in LPS-treated zebrafish. However, PRF significantly reduced the cell death in a concentration-dependent manner, although this effect was not observed the lowest concentration (25 μg mL-1).
In this study, we demonstrated the anti-inflammatory activity of PRF from EPB in LPS-induced RAW264.7 cells. Furthermore, we show that PRF reduced the pro-inflammatory mediators such as NO, iNOS, and COX-2.
Activated RAW264.7 macrophages produce NO, which plays a crucial role in physiological functions. However, overproduction of NO by iNOS can lead to cytotoxicity, inflammation, and the development of autoimmune disorders (Liu and Hotchkiss 1995, Shin et al. 2008). COX-2 is an inflammatory mediator involved in NO production (Kumar et al. 2012, Kim et al. 2013). In the present study, therefore, we investigated the inhibitory activity of PRF on NO production in RAW264.7 cells via the suppression of iNOS and COX-2 expression. We found that PRF inhibits the NO production and significantly suppresses iNOS expression, compared to COX-2 expression in LPS-induced RAW264.7 cells. These results suggest that PRF regulates the NO production via iNOS rather than COX-2.
Many studies have shown that polyphenols from
In summary, our results demonstrate that PRF inhibited the production of NO, and the expression of iNOS and COX-2 in LPS-induced RAW264.7 cells. Moreover, PRF suppressed ROS and NO generation as well as cell death in LPS-stimulated inflammation zebrafish model. Therefore, we suggest that EPB discarded during seaweed processing should be utilized as potential anti-inflammatory agents for the treatment of inflammatory diseases.