Phototrophic dinoflagellates are one of the major components in marine planktonic communities (Smayda 1997, Jeong et al. 2013a, 2013b, Park et al. 2013a). For a long time, these dinoflagellates were treated as phytoplankton, which can survive only by photosynthesis. However, in the last 2 decades, tens of phototrophic dinoflagellates have been revealed to be mixotrophic (Stoecker 1999, Jeong et al. 2005c, 2010b, 2012, Turner 2006, Burkholder et al. 2008, Kang et al. 2011); they are known to feed on diverse prey, such as heterotrophic bacteria, cyanobacteria, small flagellates, other mixotrophic dinoflagellates, and ciliates (Stoecker et al. 1997, Jeong et al. 1999, 2005b, 2005c, 2005d, 2010a, 2012, Li et al. 1999, Park et al. 2006, Seong et al. 2006, Berge et al. 2008a, 2008b, Glibert et al. 2009, Yoo et al. 2009, Kang et al. 2011). Thus, discovery of mixotrophy in phototrophic dinoflagellates increase the complexity in food webs, but help in better understanding predator-prey relationships and cycling of materials in the food webs (Jeong et al. 2010b). Some mixotrophic dinoflagellates sometimes have considerable grazing impact on populations of prey species (Jeong et al. 2005c). Recently, several new genera and / or species of phototrophic dinoflagellates have been established (Moestrup et al. 2008, 2009a, 2009b, Kang et al. 2010, Jeong et al. 2014b). To understand the eco-physiology of a phototrophic dinoflagellate and its roles in planktonic food webs of the ecosystem, the feeding ability, type of prey, feeding mechanisms, growth and ingestion rates of this phototrophic dinoflagellate need to be explored.
Recently, we found a new mixotrophic dinoflagellate Ansanella granifera in Shiwha Bay, Korea (Jeong et al. 2014a). This newly isolated, thin-walled dinoflagellate has a type E eyespot and a single elongated apical vesicle, and it is closely related to species belonging to the family Suessiaceae. Ansanella granifera has 10-14 horizontal rows of amphiesmal vesicles, comparable to Biecheleria spp. and Biecheleriopsis adriatica, but greater in number than in other species of the family Suessiaceae (Montresor et al. 1999, Kremp et al. 2005, Moestrup et al. 2009a, 2009b, Siano et al. 2009, 2010, Kang et al. 2011, Luo et al. 2013, Jeong et al. 2014a, 2014b, Takahashi et al. 2014). Unlike Biecheleria spp. and B. adriatica, A. granifera has grana-like thylakoids (Horiguchi and Pienaar 1994a, 1994b, Moestrup et al. 2009a, 2009b, Jeong et al. 2014a). Further, A. granifera lacks a nuclear fibrous connective, which is present in B. adriatica (Moestrup et al. 2009b, Jeong et al. 2014a). B. adriatica and A. granifera also show a morphological difference in the shape of the margin of the cingulum. In A. granifera, the cingular margin formed a zigzag line, and in B. adriatica a straight line, especially on the dorsal side of the cell (Moestrup et al. 2009b, Jeong et al. 2014a, Takahashi et al. 2014). The main accessory pigment is peridinin. The small subunit (SSU), internal transcribed spacer regions, and large subunit (LSU) rDNA sequences differ considerably from those of the other known genera in the order Suessiales. A. granifera has a 51-base pair fragment in domain D2 of the LSU of ribosomal RNA, which is absent in the genus Biecheleria. In the phylogenetic tree based on the SSU and LSU sequences, A. granifera belongs to the large clade of the family Suessiaceae, but a small clade containing this dinoflagellate is clearly divergent from other small clades in the family (Jeong et al. 2014a).
We established a clonal culture of A. granifera and observed its feeding behavior under high-resolution videomicroscopy in order to explore the feeding mechanisms and determine the prey species when diverse algal species were provided. We also conducted experiments to determine the effects of prey concentration on the growth and ingestion rates of A. granifera feeding on the prasinophyte Pyramimonas sp., the only algal prey, as a function of prey concentration. In addition, we estimated the grazing coefficients attributable to A. granifera feeding on Pyramimonas sp. using the ingestion rate obtained from the laboratory experiments and the abundances of predators and prey in the field. The abundances of A. granifera and Pyramimonas sp. were quantified using real-time polymerase chain reaction (PCR). The results of the present study provide a basis for understanding the feeding mechanisms and ecological roles of A. granifera in marine planktonic food webs.
The phytoplankton species were grown at 20℃ in enriched f/2 seawater media under an illumination of 20 μE m-2 s-1 of cool white fluorescent light on a 14 : 10 h light-dark cycle. The mean equivalent spherical diameter (ESD) ± standard deviation was measured by using an electronic particle counter (Coulter Multisizer II; Coulter Corporation, Miami, FL, USA). Synechococcus sp. (Genbank accession Nos. DQ023295; ESD = ca. 1 μm) were grown at 20℃ and 30-31 salinity in enriched f/2 seawater media under a 14 : 10 h light-dark cycle with 20 μE m-2s-1 of cool white fluorescent light. The heterotrophic bacterial cells that originated from a clonal culture of A. granifera were fluorescently labeled (FLB), following the method of Sherr et al. (1987). To remove any aggregated FLB, the FLB were dispersed throughout the medium using a sonicator (Bransoic cleaner 5510E-DTH; Bransoic, Danbury, CT, USA) for 10-30 min and then filtered through 3-μm poresized filter (Polycarbonate; Whatman, Dassel, Germany).
Plankton samples were collected with a water sampler from Shiwha Bay, Korea (37˚18′ N, 126˚36′ E), during September 2010, when the water temperature and salinity were 21.3℃ and 15.6, respectively (Jeong et al. 2014a). The samples were filtered gently through a 154-μm Nitex mesh and placed in 6-well tissue culture plates. A clonal culture of A. granifera was established following two serial single-cell isolations. As the concentration of A. granifera increased, A. granifera was subsequently transferred to 32, 270, and 500-mL polycarbonate (PC) bottles containing fresh f/2 seawater media. The bottles were again filled to capacity with freshly filtered seawater, capped, and placed on a shelf at 20℃ under 20 μE m-2 s-1 illumination provided by cool white fluorescent lights in a 14 : 10 h light-dark cycle.
The carbon contents of A. granifera (0.11 ng C per cell) and Pyramimonas sp. (0.04 ng C per cell) were measured using a CHN Analyzer (vario MICRO; Elementar, Hanau, Germany) and those of the other phytoplankton species were obtained from our previous studies (Jeong et al. 2010a, 2011, 2012, Yoo et al. 2010, Kang et al. 2011).
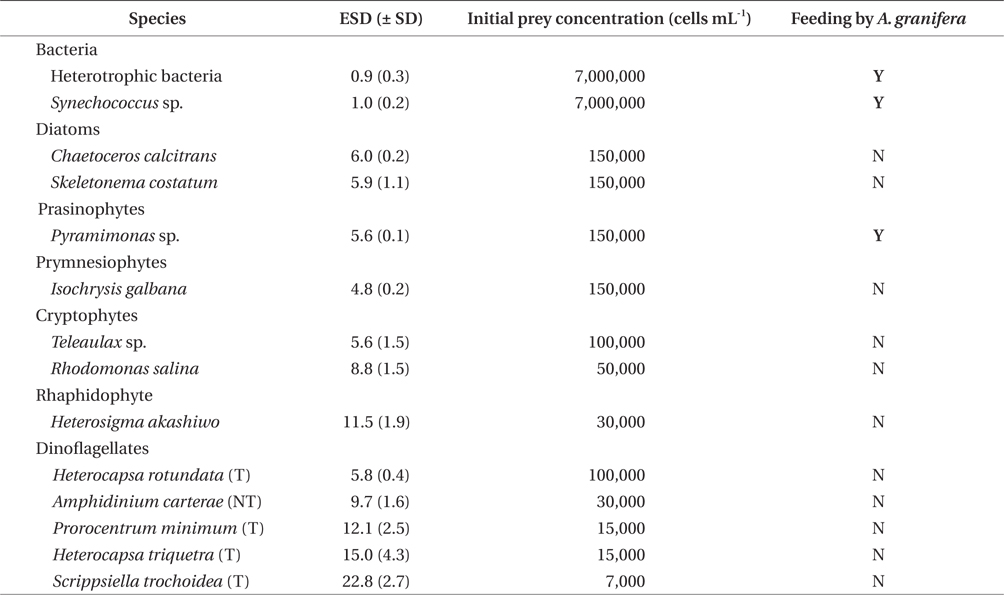
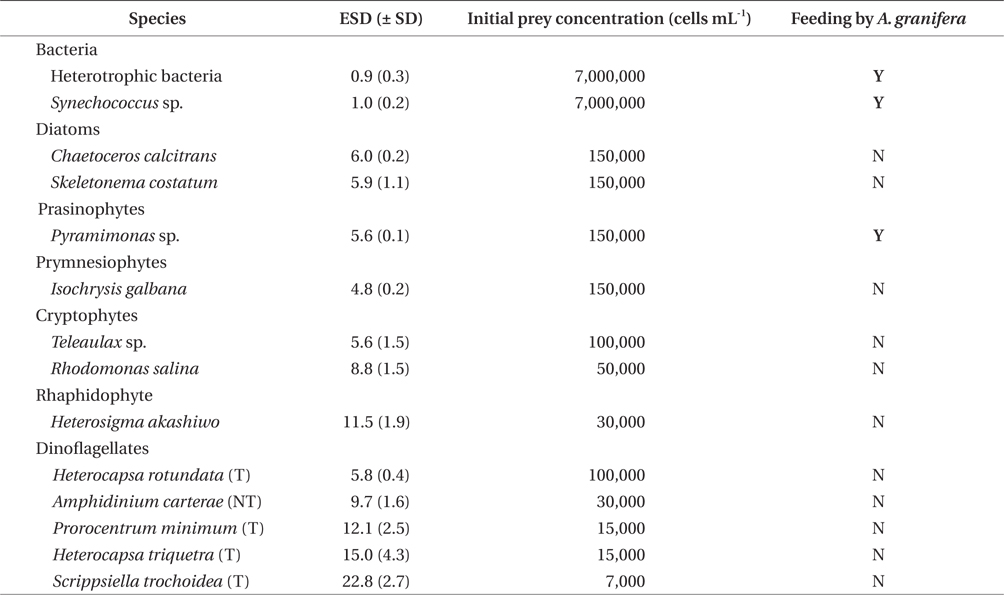
Experiment 1 was designed to investigate whether A. granifera was able to feed on different target algal species when unialgal diets of diverse algal species were provided (Table 1). The initial concentrations of each algal species offered were similar, in terms of carbon biomass. To confirm that some of the algal species were not ingested by A. granifera, additional higher prey concentrations were provided.
A dense culture (ca. 100,000-200,000 cells mL-1) of A. granifera grown photosynthetically was transferred to a 1-L PC bottle containing f/2 medium and maintained in f/2 media for 2 d. Three 1-mL aliquots were then removed from the bottle and examined using a compound microscope to determine A. granifera concentration.
In this experiment, the initial concentrations of A. granifera and each target algal species were determined by using an autopipette to deliver a predetermined volume of culture with a known cell density to the experimental bottles. Triplicate 80-mL PC bottles with mixtures of A. granifera and the target prey and duplicate predator control bottles containing A. granifera only were set up for each target algal species. The bottles were filled to capacity with freshly filtered seawater, capped, and then placed on a vertically rotating plate at 0.9 rpm and incubated at 20℃ under a 14 : 10 h light-dark cycle of cool white fluorescent light at 20 μE m-2 s-1. After 12, 24, and 48 h, a 5-mL aliquot was removed from each bottle and transferred into a 20-mL bottle. Two 0.1-mL aliquots were placed on slides and then covered with cover-glasses. Under these conditions, the A. granifera cells were alive, but almost stationary. The protoplasms of >100 A. granifera cells were carefully examined with a compound microscope and / or an epifluorescence microscope (Zeiss-Axiovert 200M; Carl Zeiss Ltd., G öttingen, Germany) at a magnification of ×100-630 to determine whether or not A. granifera was able to feed on target prey species. Images of the ingested cells of each target algal species inside A. granifera cells were taken using digital cameras mounted on the microscopes at a magnification of ×630-1,000.
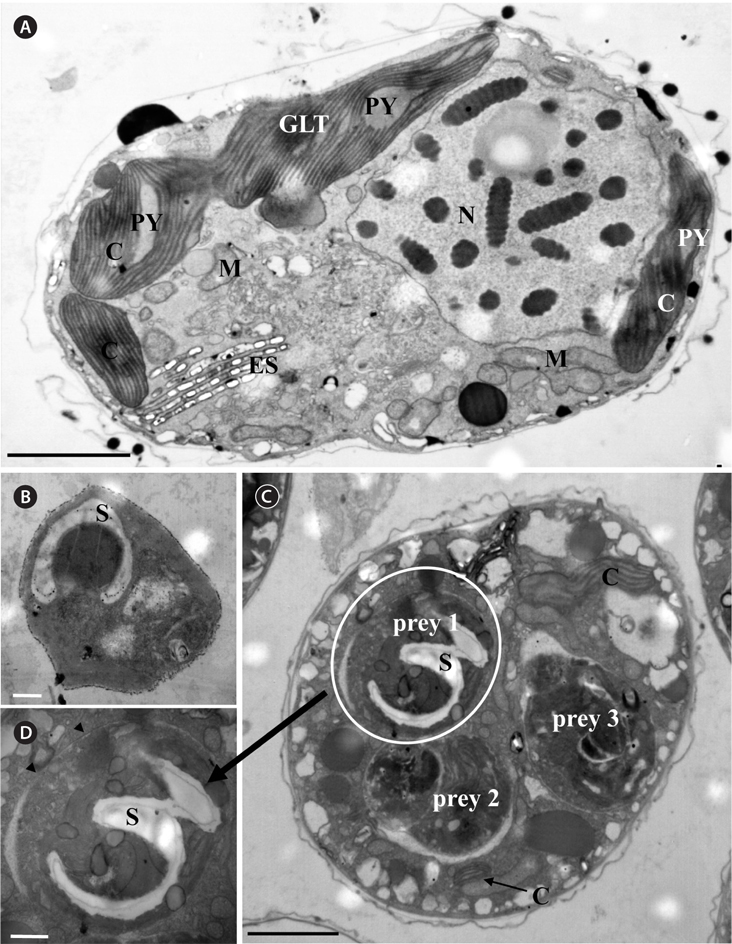
For transmission electron microscopy (TEM), each of intact Pyramimonas cells A. granifera cell grown photosynthetically, and A. granifera cell satiated with Pyramimonas sp. was transferred to a 50-mL tube and fixed in 4% (v/v) glutaraldehyde in culture medium. After 1.5-2 h, the entire contents of the tube were placed in a 50-mL centrifuge tube and concentrated at 1,610 ×g for 10 min in a Vision Centrifuge (VS-5500; Vision Scientific Co., Bucheon, Korea). The pellet from the tube was then transferred to a 1.5-mL tube and rinsed with 0.2 M sodium cacodylic acid at pH 7.4. After several rinses in the medium, the cells were post-fixed in 1% (w/v) osmium tetroxide in deionized water. The pellet was then embedded in agar. Subsequently, the pellet was dehydrated using a graded ethanol series (i.e., 50, 60, 70, 80, 90, and 100% ethanol, followed by two 100% ethanol steps). The material was embedded in Spurr’s low-viscosity resin (Spurr 1969). Sections were obtained using an RMC MT-XL ultramicrotome (Boeckeler Instruments Inc., Tucson, AZ, USA) and stained with 3% (w/v) aqueous uranyl acetate followed by 2% (w/v) lead citrate. The sections were observed using a JEOL-1010 electron microscope (JEOL Ltd., Tokyo, Japan).
Experiment 2 was designed to investigate the feeding behaviors of A. granifera when a unialgal diet of Pyramimonas sp., the only algal prey, was provided. The ingestion of these prey species by A. granifera was observed in Experiment 1. The initial concentrations of predators and prey were the same as described previously.
The initial concentrations of A. granifera and the target algal species were established using an autopipette to deliver a predetermined volume of culture with a known cell density to the experimental bottles. One 80-mL PC bottle with a mixture of A. granifera and the algal prey was set up for each target algal species. The bottle was filled to capacity with freshly filtered seawater, capped, and then well mixed. After 1-min incubation, a 1-mL aliquot was removed from the bottle and transferred into a 1-mL Sedgewick-Rafter Chamber (SRCs). By monitoring the behavior of >30 unfed A. granifera cells for each target prey species under a compound microscope and / or an epifluorescence microscope at a magnification of ×100-630, all of the feeding processes were observed. A series of images showing the feeding process of a A. granifera cell was taken using a video analyzing system (Sony DXC-C33; Sony Co., Tokyo, Japan) mounted on an epifluorescence microscope at a magnification of ×100-630.
Experiment 3 was designed to investigate the growth and ingestion rates of A. granifera. We measured the growth, ingestion, and clearance rates of A. granifera feeding on unialgal diet consisting of the optimal prey Pyramimonas sp. as a function of prey concentration.
A dense culture (ca. 32,000 cells mL-1) of A. granifera growing photosynthetically was transferred into a 1-L PC bottle containing freshly filtered seawater. The culture was transferred into one 1-L PC bottle. Three 1-mL aliquots from the bottle were counted using a compound microscope to determine the cellular concentrations of A. granifera in each bottle, and the cultures were then used to conduct experiments.
The initial concentrations of A. granifera and Pyramimonas sp. were established as described previously. Triplicate 42-mL PC experimental bottles containing mixtures of predators and prey, triplicate prey control bottles containing prey only, and triplicate predator control bottles containing predators only were set up for each predator-prey combination. To ensure similar experimental conditions, the water from A. granifera culture was filtered through a 0.7-μm GF/F filter and added to the prey control bottles at similar amounts as the volume of the predator culture added to the experiment bottles for each predator-prey combination. Next, 5 mL of f/2 medium was added to all the bottles, which were then filled to capacity with freshly filtered seawater and capped. Five milliliters of f/2 medium were added to all bottles, which were then filled to capacity with freshly filtered seawater and capped. To determine the actual initial predator and prey densities (cells mL-1) at the beginning of the experiment (A. granifera and Pyramimonas sp.: 16/237, 34/476, 51/2818, 49/5418, 96/11104, 291/71050, 525/133630) and after 2 d incubation, 5-mL aliquots were removed from each bottle and fixed with 5% Lugol’s solution, and all A. granifera cells and all or >300 prey cells in three 1-mL SRCs were enumerated. Prior to taking the subsamples, the condition of A. granifera and its prey was assessed under a dissecting microscope. The bottles were filled again to capacity with f/2 medium, capped, placed on a vertically rotating plate rotating at 0.9 rpm, and incubated at 20℃ under a 14 : 10 h light-dark cycle with 20 ℃E m-2 s-1 of cool white fluorescent light. The dilution of the cultures associated with refilling of the bottles was considered in calculating the growth and ingestion rates.
The specific growth rate of A. granifera, μ (d-1), was calculated as follows:
, where A0 is the initial concentration of A. granifera and At is the final concentration after time t. The time period was 2 d.
Data for A. granifera growth rate were fitted to the following equation:
, where μmax = the maximum growth rate (d-1), x = prey concentration (cells mL-1 or ng C mL-1), x' = threshold prey concentration (the prey concentration where μ = 0), and KGR = the prey concentration sustaining 1/2 μmax. Data were iteratively fitted to the model using DeltaGraph (SPSS Inc., Chicago, IL, USA).
Ingestion and clearance rates for 2 d were also calculated using the equations of Frost (1972) and Heinbokel (1978). The incubation times for calculating the ingestion and clearance rates were the same as for estimating the growth rate.
Ingestion rate data were fitted to a Michaelis-Menten equation:
, where Imax = the maximum ingestion rate (cells predator-1 d-1 or ng C predator -1 d-1), x = prey concentration (cells mL-1 or ng C mL-1), and KIR = the prey concentration sustaining 1/2 Imax.
After the 2-d incubation, the cell length and maximum width of A. granifera preserved in 5% acid Lugol’s solution (n = 10-30 for each prey concentration) were measured using an image analysis system on images collected with a epifluorescence microscope (AxioVision 4.5; Carl Zeiss Ltd.). The shape of A. granifera was estimated to be oval. The cell volume of the preserved A. granifera was calculated according to the following equation: volume = 4/3 π [(cell length + cell width) / 4]3.
A dense culture (ca. 50,000 cells mL-1) of A. granifera growing photosynthetically was transferred into 500-mL PC bottle. An aliquot from the bottle was added to a 50-mL cell culture flask and allowed to acclimate for 30 min. The video camera was focused on one field seen as one circle in a cell culture flask under a dissecting microscope at 20℃ and the movement of A. granifera cells was then recorded at a magnification of ×40 using a video analyzing system (SV-C660; Samsung, Seoul, Korea) and taken using a CCD camera (KP-D20BU; Hitachi, Tokyo, Japan). The mean and maximum swimming velocities were analyzed for all swimming cells seen for the first 10 min. The average swimming speed was calculated based on the linear displacement of cells in 1 s. during single-frame playback. The swimming speeds of 30 cells were measured.
By combining field data on the abundances of the predators and the target prey with the ingestion rates of the predator on the prey obtained in the present study, we estimated the grazing coefficients attributable to A. granifera feeding on co-occurring Pyramimonas sp. Data on the abundances of A. granifera and the co-occurring Pyramimonas sp. used in this estimate were obtained by analyzing the water samples taken from the waters inside and outside Shiwha Bay, Korea in 2010-2013 using realtime PCR.
The grazing coefficients (g, d-1) were calculated as:
, where CR (mL predator-1 h-1) is the clearance rate of A. granifera feeding on a target prey at a prey concentration and PC is a predator concentration (cells mL-1). The CR values were calculated as:
, where IR (cells eaten predator-1 h-1) is the ingestion rate of A. granifera feeding on the target prey and x (cells mL-1) is the prey concentration. These CR values were corrected using Q10 = 2.8 (Hansen et al. 1997) because the in situ water temperature and the temperature used in the laboratory for this experiment (20℃) were sometimes different.
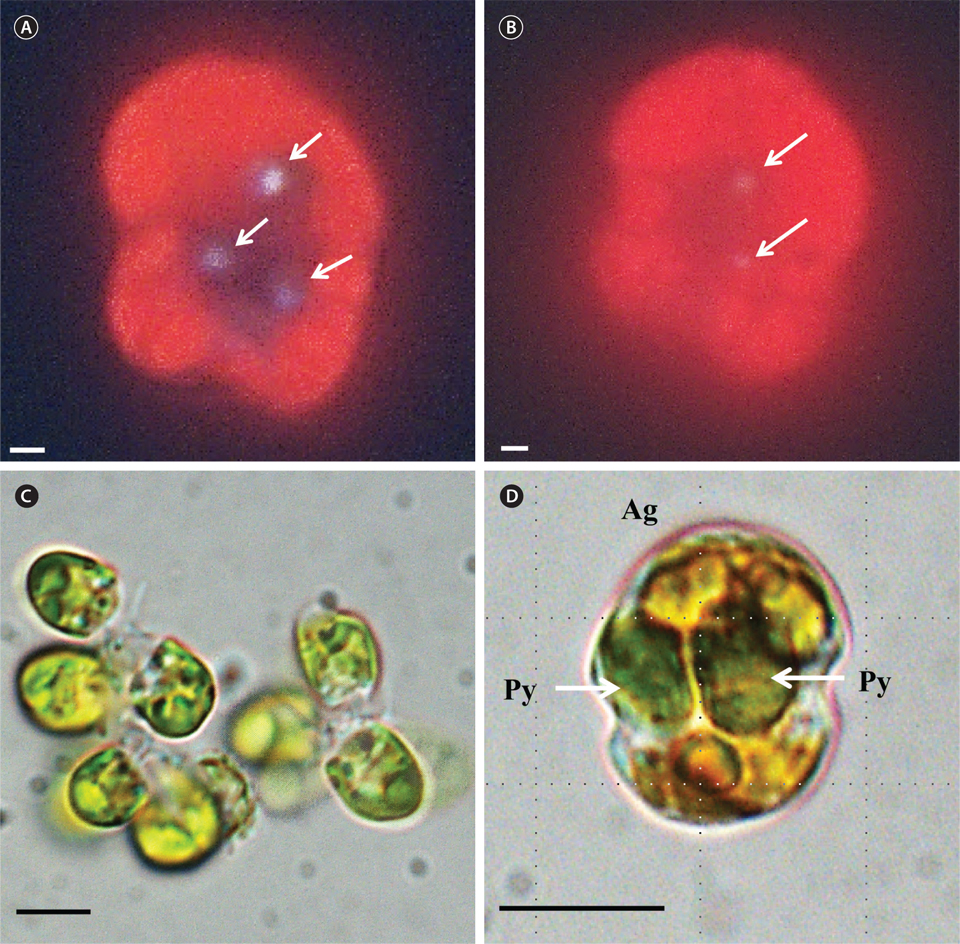
A. granifera was able to feed on heterotrophic bacteria and the cyanobacterium Synechococcus sp. (Fig. 1). In addition, among 12 algal prey species (5-23 μm in equivalent spherical diameter) offered, A. granifera ingested only Pyramimonas sp. (Table 1, Figs 1-3). TEM confirmed that A. granifera ingested Pyramimonas cells (Fig. 2). Intact Pyramimonas cells had the pyrenoids surrounded by starch. These pyrenoids with starch were conserved inside the food vacuoles of predator cells. However, it did not feed on the prymnesiophyte Isochrysis galbana, the diatoms (Skeletonema costatum and Chaetoceros calcitrans), cryptophytes (e.g., Teleaulax sp. and Rhodomonas salina), the raphidophyte Heterosigma akashiwo, the naked dinoflagellate (Amphidinium carterae), and thecate dinoflagellates (Heterocapsa rotundata, Heterocapsa triquetra, Prorocentrum minimum, and Scrippsiella trochoidea) (Table 1).
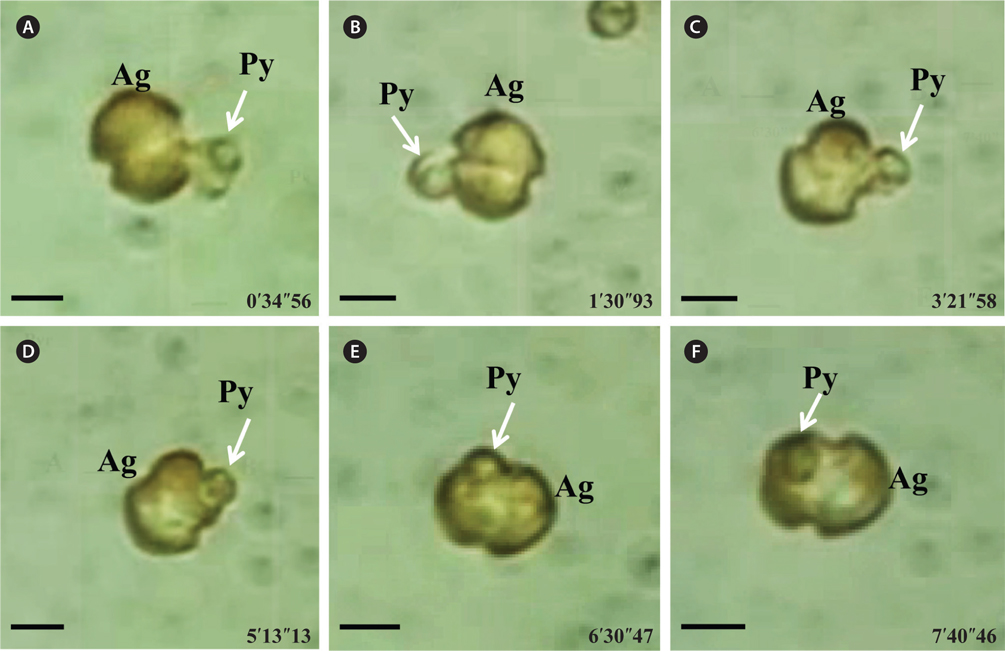
A. granifera fed on Pyramimonas sp. by engulfment after spinning a prey cell (Fig. 3). A. granifera did not try to attack the other algal species when encountering an algal cell. Furthermore, with the exception of Pyramimonas sp., it did not spin around a target cell.
With increasing mean prey concentration, the growth rate of A. granifera feeding on Pyramimonas sp. increased rapidly, but became saturated at a concentration of 434 ng C mL-1 (10,845 cells mL-1) (Fig. 4). When the data were fitted to Eq. (2), the maximum specific growth rate (i.e., mixotrophic growth) of A. granifera feeding on Pyramimonas sp. was 1.426 d-1 at 20℃ under a 14 : 10 h light-dark cycle of 20 μE m-2 s-1, while its growth rate (i.e., phototrophic growth) under similar light conditions without added prey was 0.391 d-1. The KGR (i.e., the prey concentration sustaining 1/2 μmax) was 148 ng C mL-1 (3,690 cells mL-1).
With increasing mean prey concentration, the ingestion rate of A. granifera feeding on Pyramimonas sp. increased rapidly, but slightly at concentrations ≥306 ng C mL-1 (7,649 cells mL-1) (Fig. 5). When the data were fitted to Eq. (3), the maximum ingestion rate of A. granifera feeding on Pyramimonas sp. was 0.973 ng C predator-1 d-1 (24.3 cells grazer-1 d-1) and KIR (the prey concentration sustaining 1/2 Imax) was 198 ng C mL-1 (4,958 cells mL-1). The maximum clearance rate of A. granifera feeding on Pyramimonas sp. was 0.4 μL grazer-1 h-1.
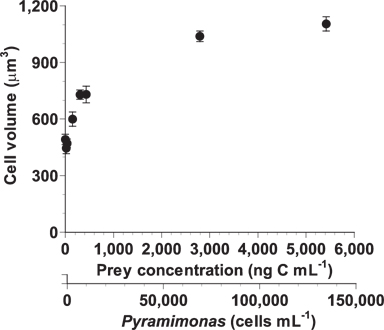
After a 2-d incubation, the mean cell volumes of A. granifera fed on Pyramimonas sp. at the lowest mean prey concentrations of 19-38 ng C mL-1 (446-471 μm3) was comparable to those that were starved (492 μm3) (Fig. 6). The cell volume increased rapidly up to 1,039 μm3 at the mean prey concentration of 2,794 ng C mL-1 and then slowly up to 1,104 μm3 at the mean prey concentration of 5,419 ng C mL-1.
A. granifera swam with alternating slow moving and very quick moving swims. The average (± standard error, n = 30) and maximum swimming speeds of A. granifera in the given conditions were 802 (± 51) and 1,603 μm s-1, respectively.
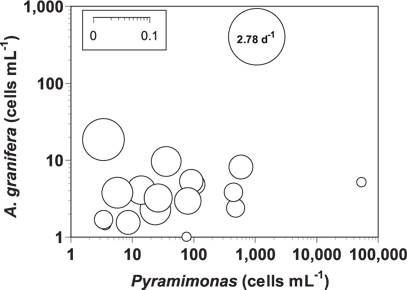
The grazing coefficients attributable to A. granifera feeding on co-occurring Pyramimonas sp. in the water samples taken in the waters of Shiwha Bay, Korea in 2010-2013 (n = 20), when the concentrations of Pyramimonas sp. and A. granifera were 3-53,243 cells mL-1 and 1-403 cells mL-1, respectively, were 0.003-2.78 d-1 (Fig. 7). The highest grazing coefficient was obtained when the concentration of Pyramimonas sp. and A. granifera were 1,053 cells mL-1 and 403 cells mL-1, respectively. In 11 of 20 samples, the grazing coefficients attributable to A. granifera feeding on co-occurring Pyramimonas sp. exceeded 0.02 d-1.
The mixotrophic dinoflagellate A. granifera fed on algal prey by engulfment. Similar-sized and shaped mixotrophic dinoflagellates Biecheleria cincta (previously Woloszynskia cincta), Gymnodinium aureolum, Karlodinium armiger, and Paragymnodinium shiwhaense, along with the heterotrophic dinoflagellates Gyrodiniellum shiwhaense, Luciella masanensis, Pfiesteria piscicida, and Stoeckeria algicida, feed on algal prey using a peduncle (Burkholder et al. 1992, Jeong et al. 2005a, 2006, 2007, 2010a, 2011, Berge et al. 2008a, 2012, Kang et al. 2011). However, the peduncle is not found inside the protoplasm of A. granifera cells. Among the algal prey tested, A. granifera was only able to feed on the prasinophyte Pyramimonas sp. (5.6 μm in ESD). In general, engulfmentfeeding dinoflagellates are able to ingest prey cells that are smaller than themselves, whereas peduncle-feeding dinoflagellates are able to feed on prey cells that are larger than themselves (Jeong et al. 2005a, 2005d, 2010a, Lim et al. 2014). The small size and engulfment feeding mechanism of A. granifera may be responsible for its feeding on only small Pyramimonas sp. The heterotrophic dinoflagellate S. algicida is able to feed only on the raphidophyte H. akashiwo, while the sister species, Stoeckeria changwonensis is able to feed on diverse prey species (Jeong et al. 2005a, Lim et al. 2014). However, the maximum growth and ingestion rates of S. algicida are greater than those of S. changwonensis. Therefore, Lim et al. (2014) suggested that diversification of prey items and feeding intensity may be traded during evolution. The maximum growth and ingestion rates of A. granifera on Pyramimonas sp. are comparable to or greater than those of other dinoflagellate predators (see next section). It will be worthwhile exploring the relationship between the number of prey species and feeding activity of other mixotrophic and heterotrophic dinoflagellates. One or few prey species may limit the period in which these predators appear and cause large fluctuations in their abundance.
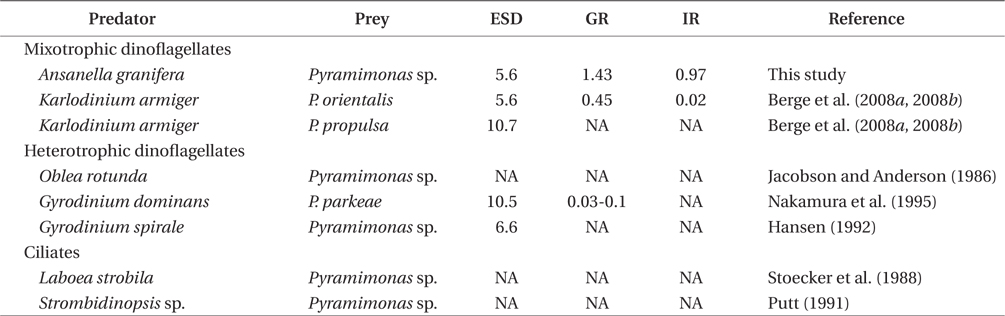
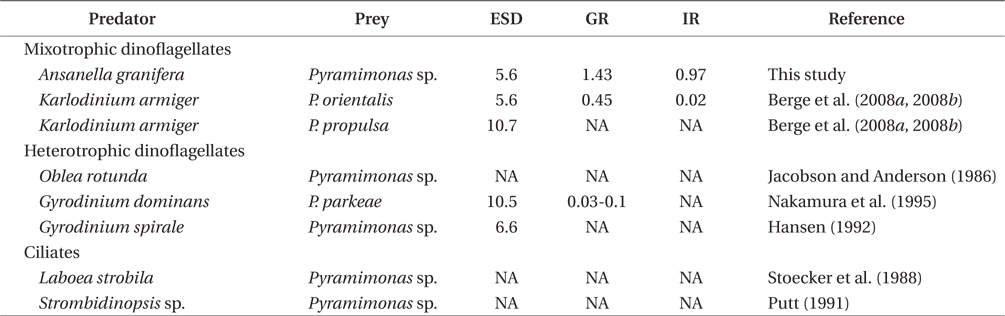
Pyramimonas spp. sometimes causes red tides or harmful algal blooms (Bird and Karl 1991, Gradinger 1996, Alonso-Rodriguez et al. 2000, Daugbjerg et al. 2000, Rodriguez et al. 2002, Kang et al. 2013). Prior to this study, K. armiger was the only mixotrophic dinoflagellate known to feed on Pyramimonas sp. (Berge et al. 2008a, 2008b). A. granifera is now one of the two mixotrophic dinoflagellates that have been reported to feed on Pyramimonas spp. (Table 2). The heterotrophic dinoflagellates Gyrodinium dominans, Gyrodinium spirale, and Oblea rotunda, and the ciliates Laboea strobili and Strombidinopsis sp. are known to feed on Pyramimonas spp. (Jacobson and Anderson 1986, Stoecker et al. 1988, Putt 1991, Hansen 1992, Nakamura et al. 1995). However, to date, positive growth rates of only G. dominans, K. armiger, and A. granifera have been reported. Therefore, during Pyramimonas blooms, G. dominans, K. armiger, and A. granifera may be abundant. To predict the population dynamics of G. spirale, O. rotunda, L. strobili, and Strombidinopsis spp. during Pyramimonas blooms, it will be worthwhile measuring the growth and ingestion rates of these predators on Pyramimonas spp.
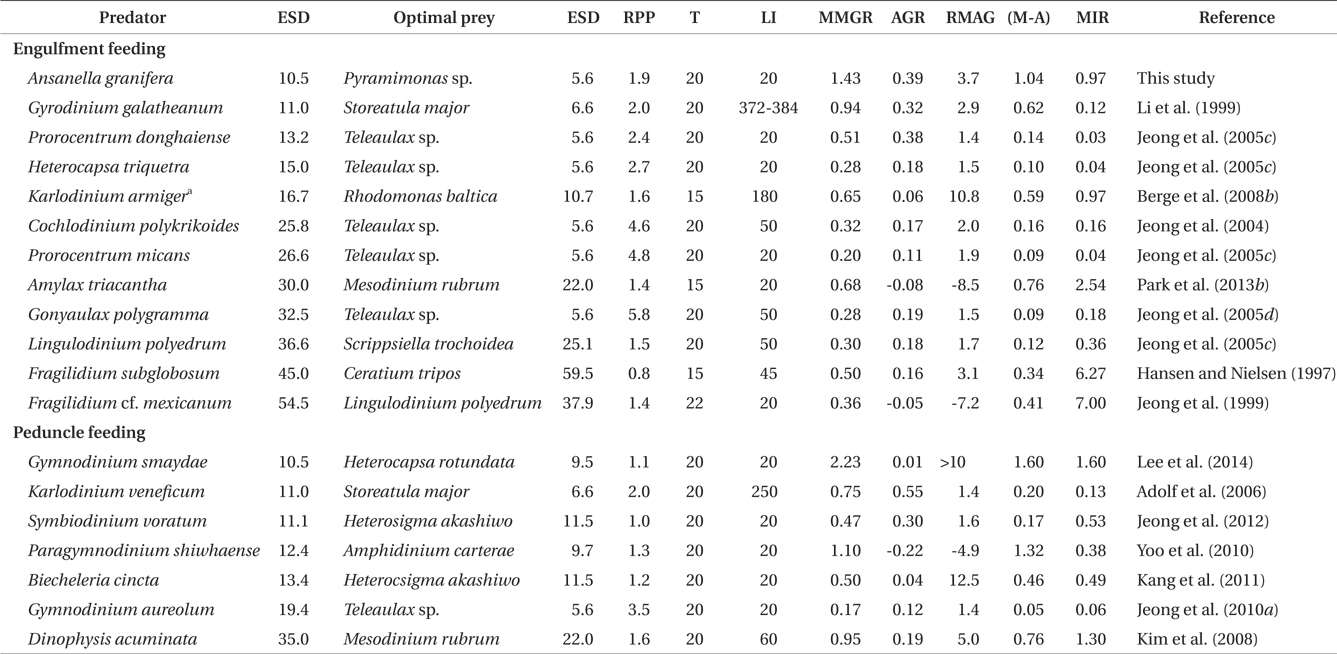
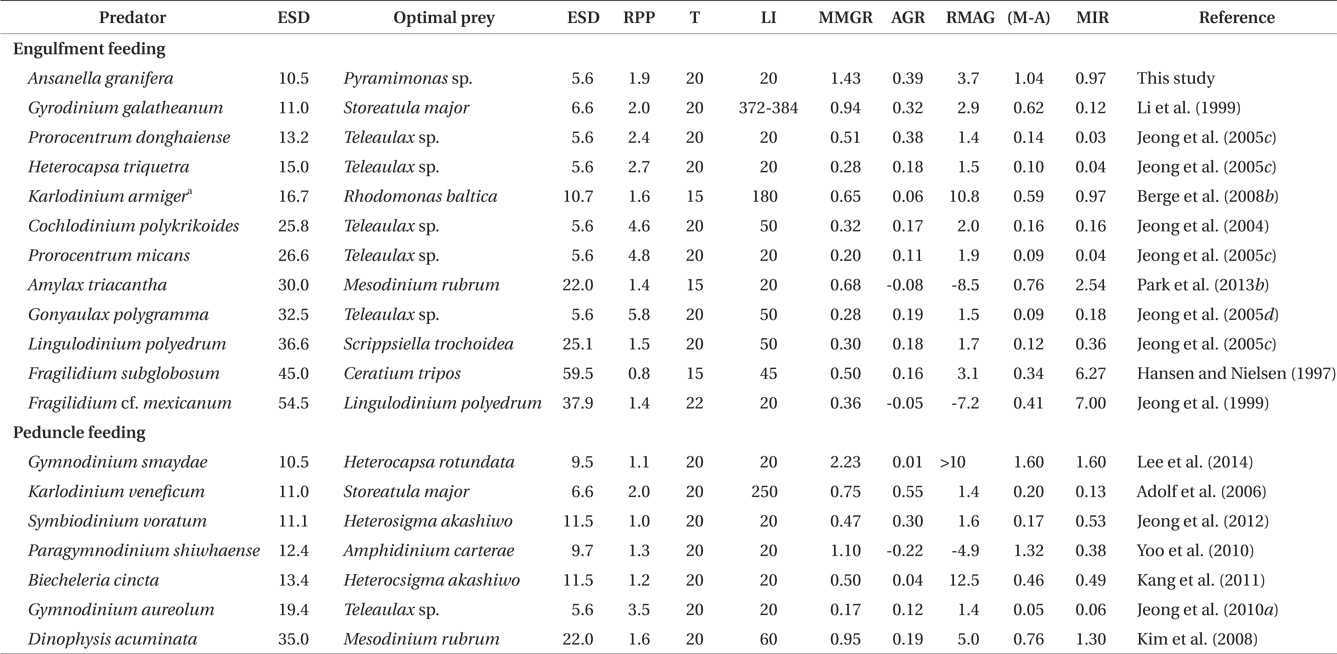
The maximum growth rate of A. granifera feeding on Pyramimonas sp. (1.426 d-1) is much greater than that of K. armiger feeding on Pyramimonas orientalis (0.45 d-1) (Table 2). The maximum ingestion rate of A. granifera feeding on Pyramimonas sp. (0.973 ng C predator-1 d-1) is much greater than that of K. armiger on P. orientalis (0.02 ng C predator-1 d-1). The cell size of A. granifera (10.5 μm in ESD) is smaller than that of K. armiger (16.7 μm). Therefore, the much higher ingestion rate of A. granifera feeding on Pyramimonas sp. and smaller cell size may be partially responsible for this greater growth rate.
The ratio of the mixotrophic growth rate (1.426 d-1) relative to the autotrophic growth rate (0.391 d-1) of A. granifera feeding on Pyramimonas sp. at 20℃ under a 14 : 10 h light-dark cycle of 20 μE m-2 s-1, 3.7, is greater than that of any other mixotrophic dinoflagellates except K. armiger on Rhodomonas baltica, Dinophysis acuminata on Mesodinium rubrum, and B. cincta (previously W. cincta) on H. akashiwo (Table 3). Furthermore, the difference between autotrophic and mixotrophic growth rates of A. granifera feeding on Pyramimonas sp., which is 1.04, is greater than any other mixotrophic dinoflagellate except P. shiwhaense on A. carterae (Table 3). Therefore, A. granifera can acquire growth materials and energy through feeding much more than photosynthesis compared to other mixotrophic dinoflagellates, except a few species. Mixotrophy in A. granifera may be a critical strategy in increasing its population.
The maximum growth rate of A. granifera feeding on the optimal prey obtained under a 14 : 10 h light-dark cycle of 20 μE m-2 s-1 (1.426 d-1) is lower than that of Gymnodinium smaydae (2.23 d-1) (Lee et al. 2014), but higher than that of any other mixotrophic dinoflagellate so far reported (0.20-1.10 d-1) at diverse light intensities (Table 3). Even though G. smaydae has the highest mixotrophic growth rate among dinoflagellates, it cannot grow well photosynthetically. High mixotrophic and autotrophic growth rates enable A. granifera to slowly increase its population when the concentration of Pyramimonas spp. is low, but rapidly increase when that of Pyramimonas spp. is high.
The maximum ingestion rate of A. granifera feeding on Pyramimonas sp. (0.97 ng C predator-1 d-1) is also greater than that of the mixotrophic dinoflagellates Karlodinium veneficum, Gyrodinium galathenum, P. shiwhaense, Prorocentrum donghaiense, which are similar in size to A. granifera (0.03-0.38 ng C predator-1 d-1) (Table 3). A. granifera feeds on only Pyramimonas sp., while the other mixotrophic dinoflagellates can feed on diverse algal prey species (Li et al. 1999, Jeong et al. 2005d, Adolf et al. 2006). Therefore, A. granifera may have adapted to feed on Pyramimonas sp. unlike other mixotrophic dinoflagellates.
When the data on maximum mixotrophic and ingestion rates of A. granifera and other mixotrophic dinoflagellates so far reported were analyzed, the maximum ingestion rates of all mixotrophic dinoflagellates feeding on the optimal prey species were significantly correlated with the size of the predator (p < 0.01, a linear regression ANOVA) (Fig. 8). Furthermore, the maximum ingestion rates of engulfment feeders are also significantly correlated with the size of the predator (p < 0.01, a linear regression ANOVA), but those of the peduncle feeders were not significantly correlated (p > 0.1). This suggests that large engulfment feeding mixotrophic dinoflagellates may ingest more prey cells than smaller ones, but peduncle feeding mixotrophic dinoflagellates may not. The maximum mixotrophic growth rates of all mixotrophic dinoflagellates feeding on the optimal prey species were not significantly correlated with the size of the predator (p > 0.1, a linear regression ANOVA). Difference in nutritional values of the optimal prey species for each predator and / or growth efficiency may have contributed to the absence of a significant correlation.
[Fig. 8.] The maximum mixotrophic growth (MMGR) (A) and ingestion rates (MIR) (B) of mixotrophic dinoflagellates feeding on optimal prey species by engulfment (close circles) and peduncle (open triangles) as a function of the size (equivalent spherical diameters [ESD, ℃m]) of the predator (see Table 3). The equation of the regression was MIR (ng C predator-1 d-1) = 0.1197 × (ESD of predator) - 1.592, r2 = 0.579 for all mixotrophs (n = 18, p < 0.01); MIR (ng C predator-1 d-1) = 0.1371 × (ESD of predator) - 2.07, r2 = 0.604 for engulfment feeders (n = 16, p < 0.01). MIR of the peduncle feeders and MMGR were not significantly correlated with ESD of predators (p > 0.1). Ag, Ansanella granifera; At, Amylax triacantha; Bc, Biecheleria cincta; Cp, Cochlodinium polykrikoides; Da, Dinophysis acuminata; Fm, Fragilidium cf. mexicanum; Fs, Fragilidium subglobosum; Ga, Gymnodinium aureolum; Gg, Gyrodinium galatheanum; Gp, Gonyaulax polygramma; Ht, Heterocapsa triquetra; Ka, Karlodinium armiger; Kv, Karlodinium veneficum; Lp, Lingulodinium polyedrum; Pd, Prorocentrum donghaiense; Pm, Prorocentrum micans; Ps, Paragymnodinium shiwhaense; Sv, Symbiodinium voratum.
The average and maximum swimming speeds of A. granifera in the given conditions, 802 and 1,603 μm s-1, respectively, are greater than those of any other mixotrophic dinoflagellate. These values are greater than those of the dinoflagellate Cochlodinium polykrikoides, the fastest mixotrophic dinoflagellate previously reported (1,063 and 1,449 μm s-1, respectively). Thus, A. granifera appears to be the fastest mixotrophic dinoflagellate reported to date. Mixotrophic dinoflagellates can increase their populations by migrating between well-lit surface water and eutrophicated bottom water (Eppley and Harrison 1975, Park et al. 2001). Thus, the ability of fast swimming may enable A. granifera to reach depths deeper than those reached by other mixotrophic dinoflagellate in vertical migration. When the thermocline is located deep, A. granifera may have an advantage over other competitors in meeting eutrophicated bottom water.
The grazing coefficients attributable to A. granifera feeding on co-occurring Pyramimonas sp. obtained in the present study were up to 2.78 d-1 (i.e., 93% of the population of Pyramimonas sp. was removed by A. granifera populations in 1 d). In 11 of 20 samples, the grazing coefficients attributable to A. granifera feeding on co-occurring Pyramimonas sp. exceeded 0.02 d-1 (i.e., ≥2% of the population of Pyramimonas sp. was removed by A. granifera populations in 1 d). Therefore, A. granifera has the potential to sometimes have a considerable grazing impact on populations of co-occurring Pyramimonas sp.








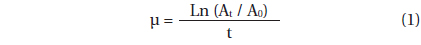
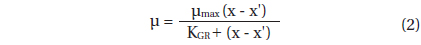
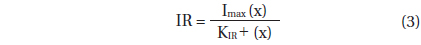
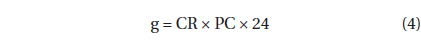
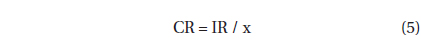
![Specific growth rate of Ansanella granifera feeding on Pyramimonas sp. as a function of mean prey concentration (x, ng C mL-1). Symbols represent treatment means ± 1 standard error. The curve is fitted by a Michaelis-Menten equation [Eq. (2)] using all treatments in the experiment. Growth rate (d-1) = 1.426 [(x + 22.03) / (147.6 + [x + 22.03])], r2 = 0.854.](http://oak.go.kr/repository/journal/13605/JORHBK_2014_v29n2_137_f004.jpg)
![Ingestion rate of Ansanella granifera feeding on Pyramimonas sp. as a function of mean prey concentration (x, ng C mL-1). Symbols represent treatment means ± 1 standard error. The curve is fitted by a Michaelis-Menten equation [Eq. (3)] using all treatments in the experiment. Ingestion rate (d-1) = 0.973 [x / (198.3 + x)], r2 = 0.948.](http://oak.go.kr/repository/journal/13605/JORHBK_2014_v29n2_137_f005.jpg)
![The maximum mixotrophic growth (MMGR) (A) and ingestion rates (MIR) (B) of mixotrophic dinoflagellates feeding on optimal prey species by engulfment (close circles) and peduncle (open triangles) as a function of the size (equivalent spherical diameters [ESD, ℃m]) of the predator (see Table 3). The equation of the regression was MIR (ng C predator-1 d-1) = 0.1197 × (ESD of predator) - 1.592, r2 = 0.579 for all mixotrophs (n = 18, p < 0.01); MIR (ng C predator-1 d-1) = 0.1371 × (ESD of predator) - 2.07, r2 = 0.604 for engulfment feeders (n = 16, p < 0.01). MIR of the peduncle feeders and MMGR were not significantly correlated with ESD of predators (p > 0.1). Ag, Ansanella granifera; At, Amylax triacantha; Bc, Biecheleria cincta; Cp, Cochlodinium polykrikoides; Da, Dinophysis acuminata; Fm, Fragilidium cf. mexicanum; Fs, Fragilidium subglobosum; Ga, Gymnodinium aureolum; Gg, Gyrodinium galatheanum; Gp, Gonyaulax polygramma; Ht, Heterocapsa triquetra; Ka, Karlodinium armiger; Kv, Karlodinium veneficum; Lp, Lingulodinium polyedrum; Pd, Prorocentrum donghaiense; Pm, Prorocentrum micans; Ps, Paragymnodinium shiwhaense; Sv, Symbiodinium voratum.](http://oak.go.kr/repository/journal/13605/JORHBK_2014_v29n2_137_f008.jpg)