Graphene, the thinnest known material in the cosmos, exhibits many outstanding properties, like extremely high strength (~130 GPa) and modulus (~1000 GPa) [1], high thermal conductivity (~5000 W/(m3∙K)) [2], and very high electrical conductivity (~6000 S/cm) [3], all due to its sp2-hybridized carbon atoms.
There have been many studies on graphene synthesis methods; the most common synthesis method is a top-down processing of graphite to graphene oxide (GO) by strong oxidation and mechanical exfoliation [4]. GO is an insulator by nature due to an abundance of oxygen-functional groups, but it can be reduced chemically or thermally to form electrically conductive graphene materials.
Graphene has been used as a nanofiller in a wide range of polymer matrices including polystyrene [5], polyurethane [6], polypropylene [7], polycarbonate [8], poly(vinyl alcohol) [9], and so on. Graphene based nanocomposites have been investigated for their superior electrical, thermal, and mechanical properties [10-12]. The incorporation of various nano-sized reinforcements into an epoxy matrix can maximize the advantages of structural composites [13-15]. Remarkable improvements in physical and mechanical properties of polymer composites have been reported upon the addition of a small amount of graphene [16-18].
In order to enhance the properties of graphene based nanocomposites, improving the dispersion of the graphene in polymer has remained the most important issue to be resolved. It is well known that graphene will normally become entangled due to intermolecular van der Waals forces and will aggregate in the polymer matrix. The resulting poor dispersion of graphene significantly lowers its efficiency as a reinforcement, and in particular, can lead to a different impact on the mechanical properties of polymer composites. A variety of processing methods, including in situ polymerization, melt mixing, and solution blending, have been proposed to disperse graphene in polymer matrix [19,20].
In this work, we prepared chemically reduced GO (RGO) for use as a mechanically enhancing filler in an epoxy matrix. The nanocomposites of epoxy resin with RGO were prepared by thermal curing process. The flexural properties of the RGO/ epoxy nanocomposites were investigated as a function of RGO loading amount.
2.1. Materials and sample preparation
GO was produced using the Hummers’ method as a starting material for graphene [21]. The chemically reduced GO was prepared as follows: 1) 1 g of GO was dispersed into distilled water using ultra-sonication; 2) 1 g of hydroquinone was added to the GO solution, and the mixture was heated to 150℃ for 24 h; 3) the GO solution was thoroughly dried at 80℃ in a vacuum oven after washing with distilled water and ethanol at a ratio of 1:1.
The epoxy polymer used in this study was diglycidyl ether of bisphenol A, supplied by Kukdo Chem. of Korea (YD-128, an epoxide equivalent weight of 185-190 g/eq, a density of approximately 1.16 g/cm3 at 25℃). 4,4›-Diaminodiphenyl methane was used as a curing agent for the epoxy polymer. The epoxy polymer and RGO powder were fed into a beaker, with ultra-sonication at 80℃ for 12 h to enhance the dispersion state of the RGO in the epoxy matrix. The composites were mixed with RGO at the weight percent loading range of 0-0.5 wt%. The stoichiometric amount of curing agent was added to the RGO/ epoxy mixtures. The mixtures were poured into a designed mold and degassed at 120℃ for 20 min in a vacuum oven. Then, the curing processes were performed, at 120℃ for 1 h and 150℃ for 2 h. Finally, samples were post-cured at 180℃ for 1 h in a convection oven.
The micro-structural characteristics of the GO and RGO were investigated using X-ray diffraction (XRD, Model D2 Phaser, BRUKER Co.). The curing behaviors were monitored by differential scanning calorimetry (DSC, Model DSC 200, NETZSCH Co.) from 50 to 250℃ at a heating rate of 10℃/min under an N2 flow. The flexural properties were measured by an Instron universal testing machine (UTM, Model LR5K, LLOYD Co.) according to ASTM D-790. The average values were obtained from the results of five tests. The fracture surfaces of RGO/epoxy composites after the flexural stress test were observed by scanning electron microscopy (SEM, Model S-4200, Hitachi Co.).
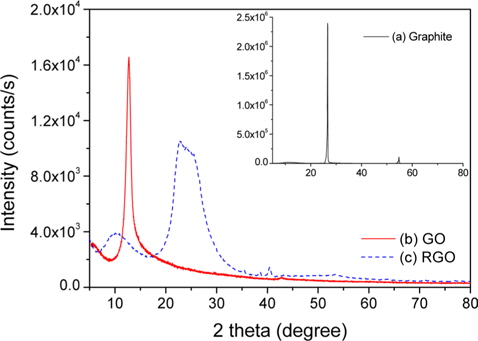
XRD patterns of graphite showed an intensive diffraction (002) peak at 2 theta = 26.6°, reflecting a
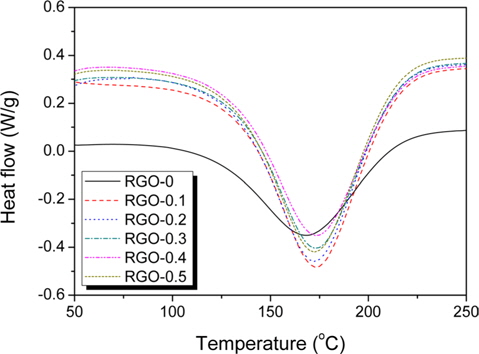
DSC curves of the RGO/epoxy mixtures are shown in Fig. 2, Grapheneinvestigating the curing behaviors of the RGO/epoxy nanocomposites. All samples showed a single exothermic peak at a high temperature, more than 150℃, originating from the decomposition of functional groups in the epoxy-based composites [18]. The main exothermic peaks of RGO/epoxy nanocomposites were observed to be at a higher temperature than those for neat-epoxy composites. It was confirmed that this was due to the steric hindrance effect on the curing process with the addition of RGO particles. The higher volume fraction of RGO increased the surface area in contact with the epoxy matrix, which could anchor the chain mobility [2223].
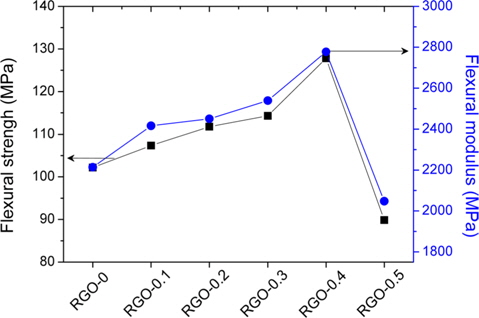
The effect of the RGO loading amounts on the mechanical properties of RGO/epoxy nanocomposites was investigated with flexural measurements. As shown in Fig. 3, the enhancement effect produced by the presence of RGO can be clearly distinguished. The flexural properties increased with increasing RGO loading amounts up to 0.4 wt%, and then decreased. The flexural strength and modulus of RGO-0.4 was determined to be 102 and 2210 MPa, respectively. It was found that the enhancement of flexural properties was strongly related to the interfacial adhesion between the RGO and epoxy matrix. The overloading of RGO (RGO-0.5) produced poor flexural properties, resulting from the aggregation of RGO in the epoxy matrix. Excessive interfacial contacts, between both the RGO/RGO and RGO/matrix, weaken the flexural properties of RGO/epoxy nanocomposites [24]. From the results, we found that the appropriate amount of RGO led to a superior reinforcement effect in the matrix.
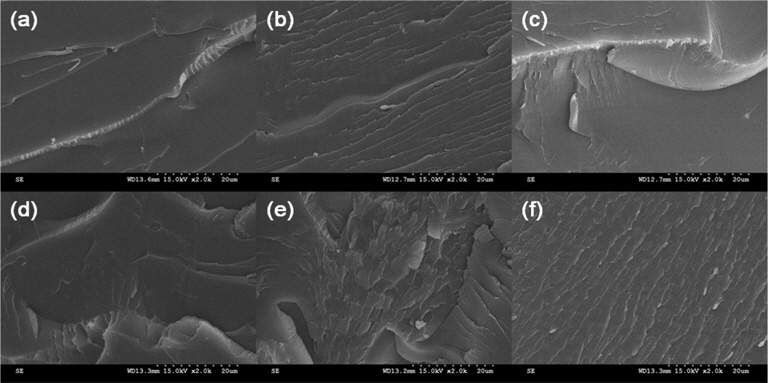
To understand the influence of added RGO on the mechanical properties of the epoxy nanocomposites, the fracture surfaces of the samples after impact tests were characterized with SEM, as shown in Fig. 4. A smooth surface (Fig. 4) was observed in the RGO-0 sample. The fracture surfaces of the RGO/epoxy nanocomposites appeared rougher and more varied in comparison with RGO-0, indicating that higher fracture energy was required [25,26]. However, the sample with excessive RGO loading, RGO-0.5, exhibited an agglomeration of RGO and some defects in the matrix. It was found that in this case the RGO had acted as stress concentrators and reduced the strengthening effects of RGO in the matrix [26].
In this work, we prepared chemically RGO for use as a mechanically enhancing filler in an epoxy matrix. The mechanical properties of the RGO/epoxy nanocomposites were investigated as a function of RGO loading amount. From the results, it was found that the flexural strength and modulus of RGO/epoxy nanocomposites were ~24.5% and ~25.7% greater, respectively, than those of the neat epoxy composites, when the added RGO amount was 0.4 wt% .