Surgical interventions including iliac autograft and allograft are necessary to reconstitute large bone defects resulting from trauma, age-associated bone loss, and occasionally, primary and secondary cancers (Zimmermann and Moghaddam, 2011). However, the supply of bone graft is limited by donor site morbidity for autograft and a shortage of natural resources for allografts. Bone tissue engineering and synthetic bone substitutes have been developed to cope with the clinical demands (Amini
Silk fibroin has been used as a suture material for a long time . Silk fibroin is a natural product with well-known biocompatibility and versatile processibility, which renders it widely applicable for tissue engineering (Vepari and Kaplan, 2007). In addition to its utility in textiles and surgical sutures, silk has been used as a biomaterial for tissue engineering of bone, tendon, eardrum, neural conduits, and blood vessels as three-dimensional (3-D) scaffolds, powders, films, and tubes (Kasoju and Bora, 2012). Three-dimensional silk scaffolds fabricated with silk fibroin alone or silk composites with diverse biomaterials and minerals have been employed in bone tissue engineering and are shown to be osteoinductive and osteoconductive in numerous cases including calvarial defect models (Park
Mesenchymal stem cells (MSCs) can be differentiated into osteoblasts, chondrocytes, adipocytes, myoblasts, and neuronal cells (Dimarino
MSCs differentiate into osteoblasts in response to combinatorial treatments using dexamethasone (DEX), ascorbic acid (AA) and β-glycerophosphate (βG) with or without BMPs in vitro (Kim
>
Preparation of silk scaffolds
A silkworm cocoon , harvested from Rural Development Administration (Suwon, Korea), was used for the experiments. The cocoon was sliced and degummed twice with 0.5% on the weight of cocoon (o.w.c.) Marseilles soap and 0.3% o.w.c. sodium carbonate solution at boiling temperature for 1 h and then washed with distilled water. The degummed cocoon was dissolved in CaCl2:H2O:ethanol (1:8:2). The silk fibroin (SF) solution was obtained after dialysis against distilled water for 4 d and then stored in a refrigerator until use (Kweon
Fibroin solutions (9% w/w concentrations) were obtained by concentrating the above fibroin solution. These solutions together with porogen (300–500 μm NaCl particles) were put into glass disks and then frozen in a −70℃ freezer for 12 h to fabricate sponge-type scaffolds 85-1 and 88-1. The composites were lyophilized and then immersed in ethanol and water to induce crystallization and to remove porogen.
Nanofibrous 3-D scaffolds were fabricated by electrospinning of 12% w/w dope solution. The dope was prepared and electrospun into a metal bath filled with methanol as a collector for SF nanofiber as described in Ki
hBMSCs (ScienCell Research Laboratories, Carlsbad, CA) were grown in αMEM (Invitrogen, Carlsbad, CA) supplemented with 16.5% fetal bovine serum (Lonza, Basel, Switzerland), 100 units/mL penicillin, and 100 μg/mL streptomycin (JBI, Korea) at 37℃ in a humidified 5% CO2 incubator. hBMSCs at ~80% confluency were subcultured by trypsinization and plating 5 × 105 cells on a 10-cm culture dish every 3–4 d.
>
Osteoblastic differentiation of hBMSCs
Osteoblastic differentiation of hBMSCs was carried out either in a 96-well plate or on the silk scaffolds. hBMSCs up to passage 10 were plated at 8,000 cells/well in a 96-well plate and treated with ODM on the next day. Alternatively, hBMSCs (5 × 104) inoculated on a silk scaffold (φ 6 mm × 3 mm thick for 10/20 and 30/50 or a quarter of φ 9 mm diameter × 1.5 mm thick for 85-1 and 88-1) were grown for a week before treatment with ODM. Three different combinations of dexamethasone (DEX), ascorbic acid (AA), and β-glycerophosphate (βG) were used for osteoblastic differentiation of hBMSCs, namely, ODM 1–50 μM AA, 10 mM βG, 100 nM DEX (Pittenger
The viability of hBMSCs seeded in a 96-well plate at 8,000 cells/well was measured by methylthiazolyldiphenyltetrazolium bromide (MTT; Sigma-Aldrich, St Louis, MO, USA) assay as described in Huynh
Alkaline phosphatase (ALP) activity was measured directly for each well in a 96-well plate (Kim
>
Alizarin S and von Kossa staining
Cells in a 96-well plate and in a silk scaffold were fixed with PBS-buffered 10% formalin for 30 min at RT. After washing with distilled water twice, the plate and scaffold were incubated in 1% Alizarin S solution or 5% silver nitrate for 30 min at RT. Then, they were washed with distilled water and dried in air. Stained Alizarin S was eluted by incubation in 100 μL of 100 mM cetylpyridinium chloride for 1 h and quantified by measuring absorbance of the eluate at 570 nm with a Multiskan GO Spectrophotometer. Cells grown in a silk scaffold were visualized by staining with 1 μg of 4′,6′-diamidino- 2-phenylindole (DAPI)/mL PBS after permeabilization with 0.2% Triton X-100.
>
In vivo bone formation assay
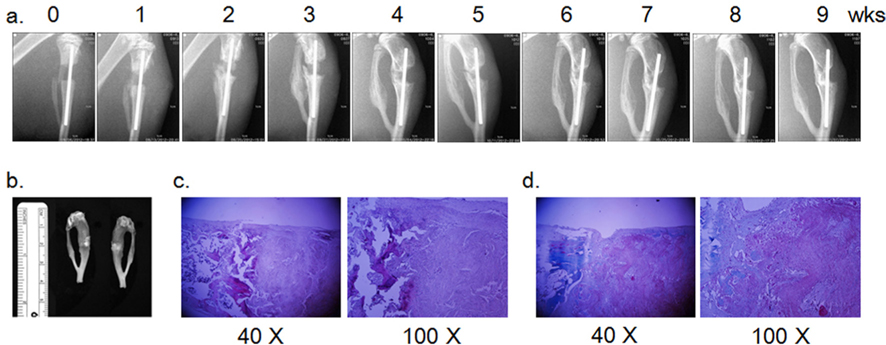
The in vivo-bone reconstitution capacity of a nanofibrous silk 3-D scaffold 3050 was assessed by implanting the scaffold into a rat tibia diaphysis defect. The tibia of the right hind limb of a rat (~350 gm male Sprague Dawley, RaonBio, Korea) anesthetized by inhalation of isoflurane (Hana Pham Co., Ltd, Korea) was exposed, and a ~3 mm longitudinal defect was generated by a sagittal saw (INT-200S, INTEC). An intramedullary nail (φ 0.9~1.2 mm, Solco Biomedical, Korea) loaded with the silk scaffold (3050) was installed into the tibia defect to fill the gap. Then, the site of surgery was closed with a black silk suture (Ailee Co., Ltd., Korea), and ceftriaxone (4 mg in 100 μL) was injected intradermally for prophylactic purpose. Bone formation was monitored weekly by radiography with EZX-60 portable dental X-ray (Genoray Co., Ltd., Korea). Animal care and operating procedures were approved by the Hallym University Medical Center Institutional Animal Care and Use Committee, Korea (HMC2012-0-1026-1).
>
H & E and Masson trichome stain
Nine wks post operation, the lower limb was surgically extirpated, and soft tissue was removed with a scalpel. After removal of the intramedullary nail, the tibia was decalcified by incubation in Calci-clear Rapid (National Diagnostics, Atlanta, GA) at room temperature overnight. Paraffinembedded tissue was processed by the standard procedure of hematoxylin and eosin (H & E) staining. Masson trichrome staining was carried out with the Trichrome Stain Kit (Sigma- Aldrich) according to the manufacturer’s protocol.
>
Osteoblastic differentiation of hBMSCs
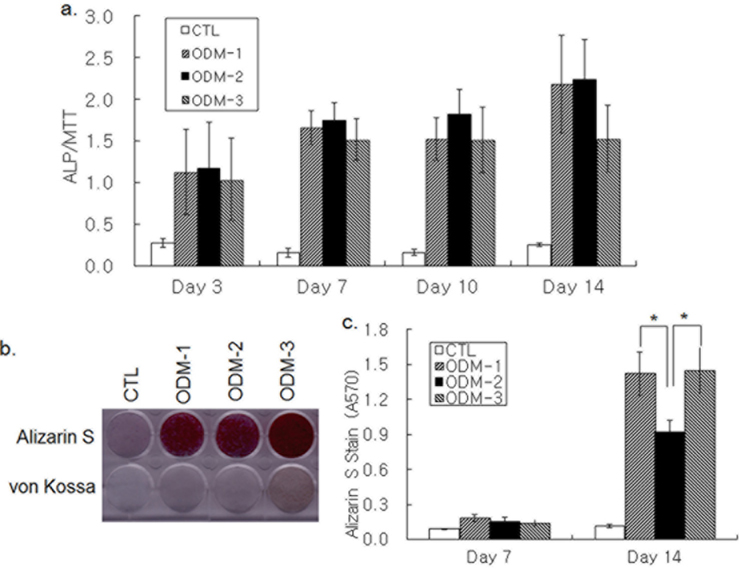
Osteoblastic differentiation was induced by treatment of hBMSCs grown in 96-well plates with three different combinations of DEX, AA, βG, and BMP-2. ALP activity was increased in 3 d of ODM treatment and kept increasing until d 14 (Fig. 1a). Although the ALP activity increase on d 14 by ODM-3 appeared lower than that by ODM-1 and ODM-2, the difference was not significant statistically. Deposition of bone mineral by differentiating hBMSCs was determined by Alizarin S and von Kossa staining. An increase in both Alizarin S and von Kossa staining was not apparent in 7 d, but all three differentiation media increased the staining intensity strongly on d 14 (Fig. 1b). Colorimetric measure of Alizarin S precipitate showed that Alizarin S staining was stronger in the ODM-1 and ODM-3-treated groups than in the ODM- 2-treated one, suggesting that ODM-2 was less efficient for calcium deposition than ODM-1 and ODM-3 (Fig. 1c). Moreover, the intensity of von Kossa staining was the strongest with ODM-3 treatment (Fig. 1b). In summary, although all three differentiation media tested were similarly effective in ALP activity increase, there was a significant difference in their potencies to induce mineral deposition.
>
Growth of hBMSC and osteoblastic differentiation on silk scaffolds
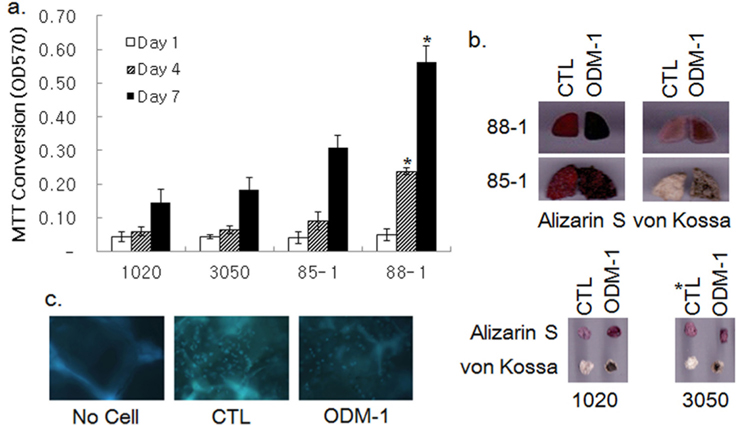
The growth of hBMSCs in various silk 3-D scaffolds was examined by MTT assay. Fibroin nanofibrous scaffolds with pores of φ 100–200 (1020) and 300–500 (3050) μm, and two sponge-type scaffolds prepared by the salt-leaching method with φ 300~500 μm pores (85-1 and 88-1) were tested for their capability to support cell growth for a wk. Cell growth in the scaffolds was attenuated until d 4 and then increased rapidly until d 7 post inoculation, except for scaffold 88-1, in which cell growth was relatively steady (Fig. 2a). Although cell growth in the 3050 scaffolds appeared to exceed that in the 1020 ones, the difference was not statistically significant. The growth of cells in the sponge-type scaffolds was ~1.5 to 3-fold higher than that in the 3050 nanofibrous scaffolds. Among the sponge-type scaffolds, the 88-1 scaffold supported cell growth better than the 85-1 scaffold did.
Osteoblastic differentiation of hBMSCs in the silk scaffolds was examined by treatment of ODM-1 followed by Alizarin S and von Kossa staining. Alizarin S and von Kossa staining demonstrated that treatment of ODM-1 induced differentiation of hBMSCs cultivated both on nanofibrous scaffolds (1020 and 3050) and sponge-type scaffolds (85-1 and 88-1; Fig. 2b). DAPI staining verified the presence of differentiated cells in scaffold 88-1 (Fig. 2c). Thus, all of the silk scaffolds tested could support growth and osteoblastic differentiation of hBMSCs in vitro.
>
In vivo bone reconstitution capacity of silk 3-D scaffolds
Since a nanofibrous silk scaffold supports calvaria bone regrowth, bone forming activity of the 3050 nanofibrous scaffold was examined in a long bone defect model of rat tibia diaphysis (Park
MSCs established from various sources, including bone marrow, dental pulp, adipose tissue, peripheral blood, and umbilical cord blood have the potential to differentiate into osteoblasts with appropriate treatments (Gamie
Attachment, growth, and differentiation of MSCs in 3-D scaffolds are required to produce bone tissue in vitro and to reconstitute bone tissue in vivo (Murphy
Although the nanofibrous scaffold 3050 supported growth and osteoblastic differentiation of inoculated MSCs in vitro, bone tissue formation was limited at the edge of the implanted 3050 scaffolds in the in vivo tibia diaphysis defect model. Unlike the long bone defect model, the nanofibrous scaffold served as a guide of bone regrowth in the rat calvaria defect model (Park
Healing a bone fracture requires firm stabilization of the fracture throughout the healing process (Marsell and Einhorn, 2011). Large animals, including sheep, rabbit, pig, and dog, have been employed in segmental bone defect model of the long bone partly because steady fixation of the fracture can be achieved with intramedullary nailing and plaster casting (Cancedda
In summary, the current study demonstrates that BMP-2- containing ODM (ODM-2) is less effective for promoting differentiation-associated mineral deposition of human MSCs than BMP-2-deficient media. Although silk scaffoldsboth in the nanofibrous and sponge formsupport growth and differentiation of MSCs in vitro, a nanofibrous silk scaffold by itself is not sufficient to reconstitute tibial defect in the rat hind limb. Since the nanofibrous scaffold is osteoconductive in the rat calvaria model and could sustain MSC ingrowth and differentiation in vitro (Park