Inflammation is a complex response to an exterior challenge or cellular injury that leads to the release of a complex array of inflammatory moderators, eventually restoring tissue structure and function. However, prolonged inflammation can be harmful, contributing to the pathogenesis of many diseases such as cancer, diabetes, arthritis, atherosclerosis, and Alzheimer’s (Makarov, 2001; Packard and Libby, 2008; Cunningham, 2012). As the primary immune cells in the brain, microglia play an important role in modulating the innate immune response to support the integrity of the central nervous system. The developing brain is insulted by a variety of factors, including inflammation, excitotoxicity, and oxidative stress. Under these conditions, activated microglia secrete excess pro-inflammatory mediators such as nitric oxide (NO) and pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 (Bozinovski et al., 2002; Silva et al., 2008). These mediators and cytokines exacerbate neuronal tissue injury and subsequent neurodegeneration. Therefore, modulation of activated microglia is a logical therapeutic strategy to inhibit neuroinflammation.
NO is primarily produced by enzymatic action of inducible nitric oxide synthase (iNOS). Expression of this enzyme as well as pro-inflammatory cytokines is regulated by the activation of nuclear factor-kappa B (NF-κB) (Bozinovski et al., 2002); the iNOS gene promoter contains an NF-κB consensus sequence. In most cell types, NF-κB is located in the cytosol as an inactive complex bound to inhibitory kappa B (IκB) family, and is activated by diverse external stimuli including inflammatory cytokines, bacterial components, and viral infection (Pahl, 1999). Lipopolysaccharide (LPS) stimulates the phosphorylation, ubiquitination, and proteasomal degradation of IκB, resulting in the translocation of NF-κB into the nucleus by dissociation of the NF-κB–IκB-α complex (Janssen-Heininger et al., 2000). The activation of NF-κB is also controlled by cellular signaling kinases such as mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase/AKT (Cantley, 2002; Jang et al., 2005). The MAPK family, including extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun NH2-terminal kinase (JNK), regulates transcription of inflammatory genes via NF-κB activation (Herlaar and Brown, 1999; Jang et al., 2005). Elucidating the detailed molecular mechanisms of this regulation is important for counteracting the harmful effects of pro-inflammatory mediators (Jana et al., 2007).
Marine macroalgae contain an abundance of polysaccharides, minerals, and polyunsaturated fatty acids known to be beneficial to human health. Recently, diverse studies have revealed that compounds such as phlorotannins (e.g., bieckol), chromenes (sargachromenol, sargaquinoic acid, and sargahydroquinoic acid), and pigments (e.g., fucoxanthin), in extracts from
LPS (
>
Plant material and preparation of MYE
>
Cell culture and sample treatment
Murine BV-2 microglial cells (ATCC, Rockville, MD, USA) were maintained in DMEM supplemented with 10% FBS, penicillin (100 units/mL), and streptomycin sulfate (100 μg/mL) in a humidified atmosphere of 5% CO2. Cells pretreated with various concentrations of MYE for 2 h were stimulated with LPS (1 μg/mL) for the indicated times.
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay using the CellTiter 96 AQueous One Solution Cell Proliferation assay kit according to the manufacturers instructions. Cells were inoculated at a density of 3 × 105 cells into 96-well plates and cultured at 37°C for 24 h. Cells were then treated with LPS (1 μg/mL) in the presence or absence of MYE at various concentrations for 24 h. The culture medium was removed and replaced with 95 μL of fresh culture medium and 5 μL of MTS solution. After 1 h, absorbance at 490 nm was measured using a microplate reader (Glomax Multi Detection System; Promega).
>
Measurement of NO and pro-inflammatory cytokines
Cells (5 × 104 cells/well) were pretreated with MYE (0-100 μg/mL) for 2 h prior to LPS treatment for 24 h. After treatment with LPS, culture media were collected after centrifugation at 2,000 g for 10 min and stored at –75°C until tested. The nitrite concentration in culture media was measured as an indicator of NO production, according to the Griess reaction (Kim et al., 1995). Levels of TNF-α and IL-6 in culture media from each group were quantitatively determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
>
Reverse transcription-polymerase chain reaction
BV-2 cells plated in a 6-well cell culture plate at a density of 3.0 × 105 cells/well were pretreated with MYE or DMSO for 1 h, then treated with LPS for 6 h. Total RNA from each group was isolated using TRIzol reagent. Five micrograms of total RNA was used for reverse transcription using oligo-dT and M-MLV reverse transcriptase. PCR was carried out using the resulting cDNA as a template under the following conditions: 25 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The PCR products were visualized by 1% agarose gel electrophoresis. Verification of PCR product of the iNOS gene was established by its predicted sizes under an ultraviolet light illuminator. The primer sequences were the following: 5'-ACC ACT CGT ACT TGG GAT GC-3' (sense), 5'-CAC CTT GGA GTT CAC CCA GT-3' (antisense) for iNOS; 5'-GAC CCC TTC ATT GAC CTC AA-3' (sense), 5'-CTT CTC CAT GGT GGT GAA GA- 3' (antisense) for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). GAPDH was used as an internal standard to evaluate the relative expression of iNOS.
To analyze nuclear localization of NF-κB in BV-2 cells, cells were cultured on glass coverslips (SPL Lifesciences Co., Pocheon, Korea) in 24-well plates for 24 h. After preincubation with MYE for 2 h, cells were stimulated with LPS (1 μg/ mL) or treated with DMSO for 30 min. Cells were fixed in 4.0% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature, then permeabilized with 0.5% Triton X-100 in PBS for 10 min. Permeabilized cells were washed with PBS and blocked with 3% bovine serum albumin (BSA) in PBS for 30 min. Thereafter, cells were incubated with anti-NF-κB polyclonal antibody diluted in 3% BSA/PBS for 2 h, rinsed three times for 5 min with PBS, and incubated with Alexa Fluor 488-conjugated secondary antibody diluted in 3% BSA/PBS for 1 h. Cells were stained with 2 μg/mL DAPI and images were captured using an LSM700 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany).
>
Preparation of cytosolic and nuclear extracts
BV-2 cells plated in 6-well cell culture plates at a density of 3 × 106 cells per well were pretreated with MYE or DMSO for 2 h and then treated with LPS for 30 min. Cells were washed twice with ice-cold PBS, scraped in PBS, and centrifuged at 12,000 g for 5 min at 4°C. Pellets were suspended in 180 μL of hypotonic buffer A (10 mM Tris-HCl, 10 mM NaCl, 3 mM MgCl2, 0.02% NaN3, 0.5 mM dithiothreitol [DTT], and 1 mM phenylmethane sulphonyl fluoride [PMSF], pH 7.4), and afterward, 20 μL of 5% Nonidet P-40 was added and stood for 5 min on ice. The mixture was centrifuged at 1,800 g for 5 min, and the supernatant (cytosolic extract) was collected. Pellets were washed with hypotonic buffer and resuspended in hypertonic buffer C (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.02% NaN3, 0.5 mM DTT, and 1 mM PMSF, pH 7.4) for 1 h on ice and centrifuged at 14,000 g for 10 min. The supernatant containing nuclear proteins was collected and stored at –70°C after determination of the protein concentration.
Cells were incubated with various concentrations of MYE for 1 h and stimulated with LPS (1 μg/mL) for varying times. Cells were washed twice with cold PBS and lysed with lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 1% Tween 20, 0.1% sodium dodecyl sulfate [SDS], 1 mM Na3VO4, 10 μg/mL leupeptin, 50 mM NaF, and 1 mM PMSF, pH 7.5) on ice for 1 h. After centrifugation at 12,000 g for 20 min, protein concentrations in the supernatants were determined, and aliquots (30 μg protein) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose membranes (GE Healthcare). Membranes were washed with Tris-buffered saline (TBS; 10 mM Tris-HCl, 150 mM NaCl, pH 7.5) supplemented with 0.05% Tween 20 (TBST) followed by blocking with TBST containing 5% nonfat milk. Membranes were incubated overnight with primary antibodies, then exposed to secondary antibodies coupled to horseradish peroxidase for 2 h and washed three times with TBST at room temperature. Immunoreactivities were determined using an ECL detection kit.
Data were expressed as means ± SDs of at least three independent experiments unless otherwise indicated. Data were analyzed using one-way analysis of variance (ANOVA), followed by pairwise Student’s
>
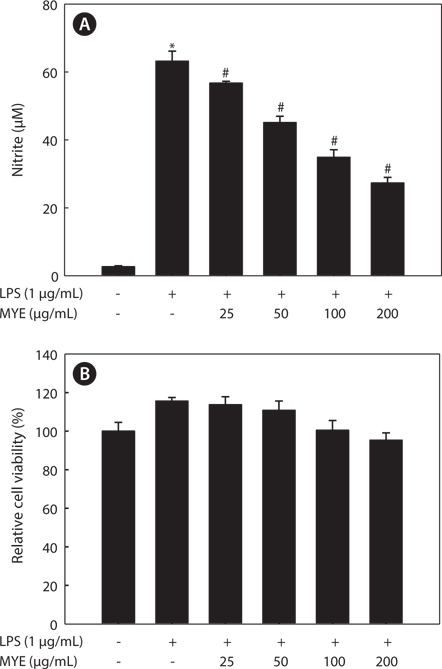
MYE suppresses NO production in LPS-stimulated BV-2 cells
Whether MYE inhibits neuroinflammation was determined by measuring NO production by LPS-stimulated BV-2 cells. Cells pretreated with various concentrations of MYE were stimulated with LPS (1 μg/mL) or treated with DMSO for 24 h. As shown in Fig. 1A, MYE significantly suppressed LPS-induced NO production in a dose-dependent manner. MTS assay revealed that MYE causes no cytotoxicity at concentrations of up to 200 μg/mL in BV-2 cells (Fig. 1B). Taken together, MYE inhibits LPS-induced NO secretion in BV-2 cells with no cytotoxicity.
>
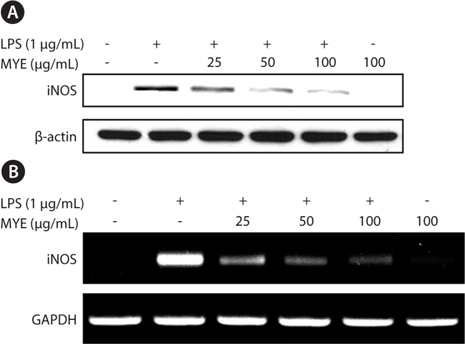
MYE inhibits inflammation-triggered expression of iNOS in BV-2 cells
We investigated whether MYE’s suppression of NO production is related to expression of iNOS by Western blotting. As shown in Fig. 2A, LPS treatment of BV-2 cells for 16 h enhanced the expression of iNOS protein. However, pretreatment with MYE led to a dose-dependent inhibition of LPS-induced iNOS protein expression in BV-2 cells; iNOS expression was almost completely suppressed at 100 μg/mL of MYE. To further examine the effect of MYE on the transcriptional regulation of iNOS expression, reverse transcription-polymerase chain reaction analysis was performed. Fig. 2B shows that MYE suppressed the expression of iNOS mRNA in a dose-dependent manner in LPS-stimulated BV-2 cells. Similar to Western blot results, 100 μg/mL of MYE strongly inhibited iNOS mRNA expression. These results indicate that MYE-mediated downregulation of iNOS expression is mainly due to its transcriptional repression in LPS-stimulated BV-2 cells.
>
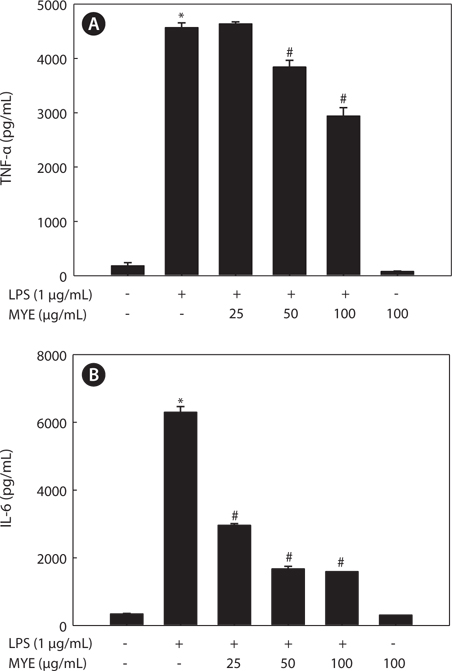
MYE inhibits LPS-induced production of TNF-? and IL-6 in BV-2 cells
Pro-inflammatory cytokines such as TNF-α and IL-6 are secreted early in the inflammatory response and are thus key markers of inflammation. We determined the effect of MYE on the levels of these cytokines in the media of LPS-stimulated BV-2 cells by ELISA following pretreatment with various concentrations of MYE for 2 h and LPS stimulation for 24 h. Stimulation of BV-2 cells with LPS induced significant increases in the levels of TNF-α (Fig. 3A) and IL-6 (Fig. 3B); pretreatment with MYE inhibited these effects in a dose-dependent manner.
>
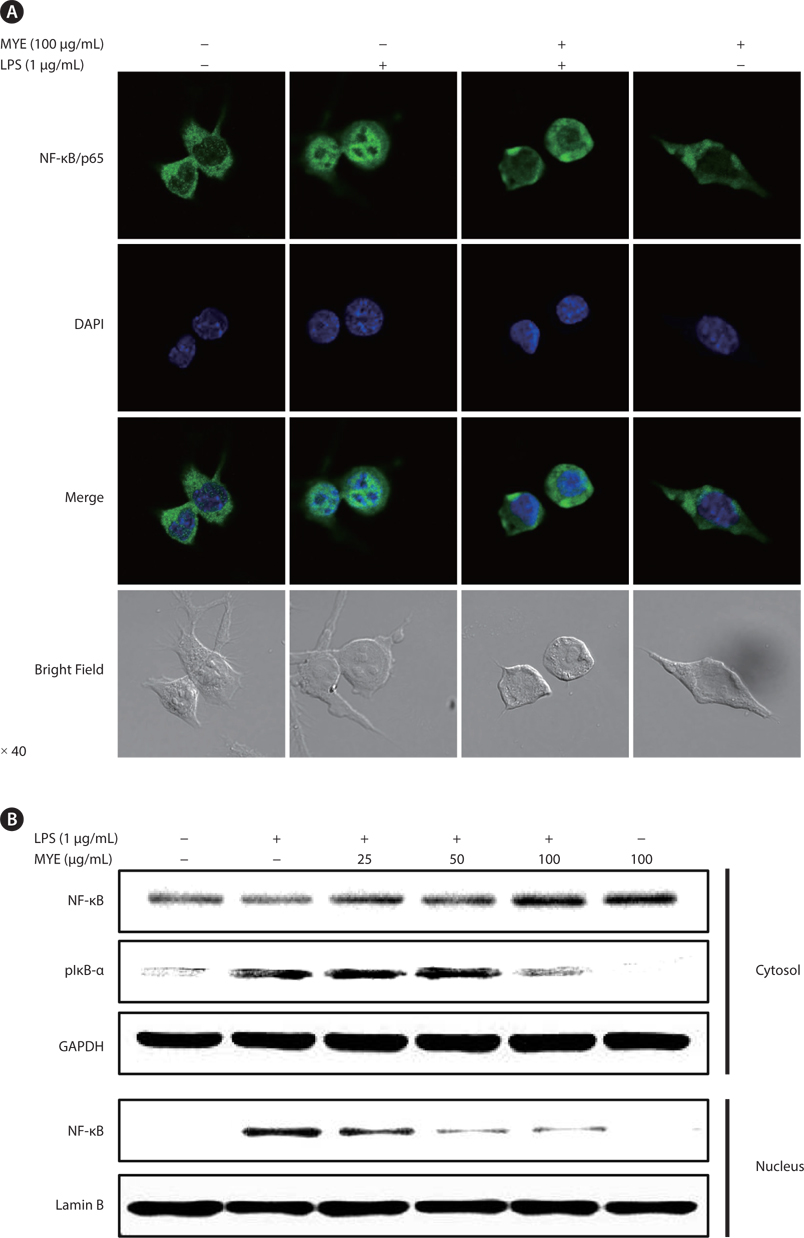
MYE suppresses nuclear translocation of NF-κB in LPS-stimulated BV-2 cells
To analyze the effect of MYE on the regulation of transcription factors during the inflammatory response, the effect of MYE on the intracellular localization of NF-κB/p65 was assessed by confocal microscopy and Western blot analysis in LPS-stimulated BV-2 cells. Immunocytochemical analysis (Fig. 4A) showed that NF-κB protein was mainly localized in the cytoplasm in unstimulated cells and was translocated into the nucleus upon stimulation with LPS. Pretreatment with MYE remarkably suppressed translocation of NF-κB regardless of LPS treatment in BV-2 cells. Subcellular fractionation and Western blot analysis confirmed LPS-induced nuclear translocation of NF-κB via degradation and phosphorylation of IκB-α, as well as MYE’s suppression of this effect (Fig. 4B). These results suggest that MYE attenuates the production of NO and pro-inflammatory cytokines at least in part through inactivation of the NF-κB pathway.
>
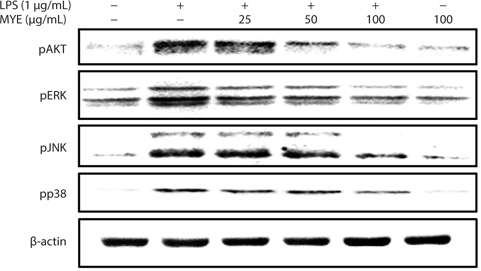
MYE prevents activation of MAPKs and AKT in LPS-stimulated BV-2 cells
To evaluate the effect of MYE on signaling associated with NF-κB activation, changes in phosphorylation of intracellular signaling proteins, including ERK, JNK, p38 MAPK, and AKT, in BV-2 cells were analyzed by Western blotting. As shown in Fig. 5, MYE inhibited phosphorylation of JNK, ERK, p38 MAPK, and AKT in a dose-dependent manner in LPS-stimulated BV-2 cells. These data suggest that inactivation of the NF-κB pathway by MYE is mediated by inhibition of MAPKs and AKT phosphorylation.
Various studies have examined the biological activities of natural compounds or extracts to develop dietary supplements or therapeutic agents due to their easy availability and low side effects. Various phytochemicals from terrestrial plants have shown a wide range of anti-inflammatory activities; moreover, many studies have elucidated the molecular mechanisms of the anti-inflammatory action of extracts from marine brown algae, such as
NO is a key inflammatory and neurotoxic mediator in neuroinflammation responsible for detrimental effects on the central nervous system in ischemia, Alzheimer's disease, and neuronal injury (Meda et al., 1995). Many studies have revealed that abnormally high levels of NO are found in various types of brain injuries and neurodegenerative diseases, which are caused by enhanced expression of iNOS (Teismann et al., 2003; Brown and Neher, 2010; Cunningham, 2012). Overexpression of iNOS was found to occur in microglial cells in rodent brains treated with LPS (Minghetti et al., 1999). Treatment with inhibitors of iNOS results in neuroprotection against LPS-induced neurotoxicity, suggesting that NO is an important mediator in neurotoxicity (Teismann et al., 2003;Oh et al., 2009). The present study demonstrated that MYE inhibits the production of NO in LPS-stimulated BV-2 cells. In addition, we determined that inhibition of NO production by MYE results from the suppression of both mRNA and protein expression of iNOS in these cells, indicating that inhibition of NO by MYE is associated with downregulation of iNOS at the transcriptional level. These data suggest that MYE may have a protective effect against the neuroinflammatory response in neurodegenerative diseases.
Activated microglia produce high levels of pro-inflammatory cytokines including IL-6 and TNF-α, which are involved in the pathogenesis of several neurodegenerative disorders (Brown and Neher, 2010; Kim et al., 2013a, 2013b). Also, excessive production of pro-inflammatory cytokines such as TNF-α and IL-6 play a critical role in acute inflammatory responses as well as chronic inflammatory diseases (Jang et al., 2005; Kim et al., 2013a). Our observation that MYE suppresses secretion of these pro-inflammatory cytokines in LPSstimulated BV-2 cells further supports the potential of MYE as a neuroprotective agent against neuroinflammation.
Transcription of iNOS and pro-inflammatory cytokine genes are mainly controlled by NF-κB, which binds to respective
Intracellular signaling proteins such as MAPKs and AKT are activated by LPS, leading to enhanced transcription of iNOS and pro-inflammatory cytokine genes in microglia (Herlaar and Brown, 1999; Skaper, 2007; Jung et al., 2010). Phosphorylation of ERK, JNK, p38 MAPK, and AKT is decreased by MYE treatment in a dose-dependent manner in LPS-stimulated BV-2 cells. Given the roles of ERK, JNK, p38 MAPK, and AKT in inflammatory processes, these kinases may be another target of MYE’s anti-inflammatory activity in LPS-stimulated BV-2 cells. These data indicate that MYE’s anti-inflammatory effects result at least in part from inhibition of the NF-κB pathway via suppressed phosphorylation of MAPKs and AKT.
In conclusion, we found that MYE inhibits the secretion of inflammatory mediators such as NO and pro-inflammatory cytokines, including TNF-α IL-6, in LPS-stimulated BV-2 microglia. Moreover, the inhibitory effect of MYE was associated with inactivation of the NF-κB pathway via reduced phosphorylation of MAPKs and AKT. However, the