Diabetes mellitus is one of the oldest diseases, and it affects millions of people all over the world [1]. The total world population with diabetes is projected to rise to 366 million in 2030 [2]. Since ancient times, the medicinal properties of plants have been investigated, and recently, new scientific developments have involved traditional practitioners in the development of modern medicine throughout the world. In view of the adverse effects associated with synthetic drugs, medicinal plants are safer, cheaper and more effective. Thus, conventional antidiabetic plants should be explored [3].
There is an increasing demand by patients to use natural products with antidiabetic activity. Furthermore, following the World Health Organization’s recommendation, investigations of hypoglycemic agents from medicinal plants have become more important [4]. The liver is regarded as the central metabolic organ in the body, with an important role in maintaining post-parandial normal glucose concentration (glyconeogenesis and glycolysis), and it is the main site of insulin clearance [5]. Diabetes, by most estimates, is now the most common cause of liver disease (hepatopathy) in the world, and liver disease, including abnormal liver enzymes, hepatocellular carcinomas, and acute liver failure, is an important cause of death. Various pharmacotherapies, such as hypoglycemic drugs, insulin, and, recently, cellular therapy, are available, but these therapies have their own limitations [6].
The available literature reveals that more than 400 Indian plant species have hypoglycemic potential [7, 8].
Around 2 kg of leaves of
Wistar rats were fasted for sixteen hours before induction of diabetes by administering a single dose of 90 mg/kg body weight of streptozotocin (STZ) in 0.9-percent normal saline injected through an intraperitoneal route [11]. Hyperglycemia was confirmed 48 hours later, and fasting serum glucose levels > 250 mg/dL were obtained by using the glucose oxidase-peroxidase method [12]. The rats were divided among five groups, each with six rats: Group 1: normal control (saline treated), Group 2: untreated diabetic rats, Groups 3 and 4: diabetic rats treated orally with PEME of
Blood samples were collected on days 0, 7, 14 and 21 after induction of diabetes from normal rats, untreated diabetic rats, rats treated with PEME of
Animals were sacrificed after 21 days, and the liver tissues were preserved in formalin (10%) for further processing and fixed in paraffin wax. Thin five-μm sections on glass slides were stained with hematoxylin and eosin dyes for histopathological evaluations. Scanning electron microscopy was performed on liver tissues from normal, untreated diabetic, PEME-treated, and metformin-treated rats. The blocks were fixed in 2.5% glutaraldehyde buffered in 0.1-M phosphate overnight at 4℃. The specimens were then washed in a phosphate buffer thrice and osmicated in 1% osmium tetroxide for 2 hours. After the specimens had been washed in buffer and dehydrated in a graded series of ethanol solutions, they were dried in a critical-point drying apparatus (Polaron SC 7620, Quorum Technologies, Sussex, United Kingdom) by using liquid carbon dioxide, were mounted on aluminum stubs, and were vacuum coated with gold palladium (Polaron SC 7620, Quorum Technologies, Sussex, United Kingdom). Coded specimens were then viewed under a scanning electron microscope (Model VEGA II L50, LSU, Tescan, Czech Republic) operated at 10 kV. The entire specimen was scanned on a monitor at a magnification of x885. For each block of tissue, an area with maximum damage was chosen and photographed.
The results are expressed as means ± standard deviations (S.D.’s). Graph pad prism version 5.04 was used for the ANOVA. That was followed by Tukey’s multiple comparison to assess the mean differences and the significance variations.
3.1. Serum biochemical parameters
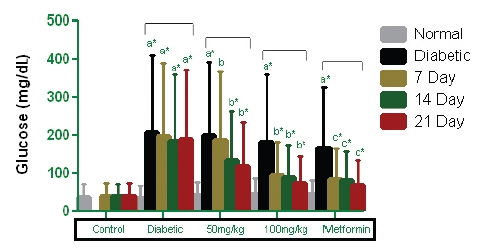
Rats with STZ-induced diabetes that had fasted exhibited a significant elevation in serum glucose levels after 48 hours. Administrations of PEME of
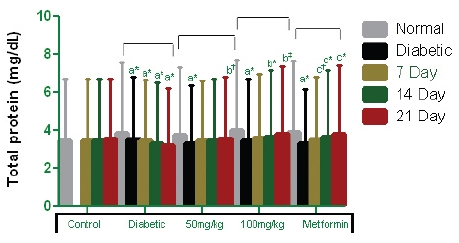
The serum total protein concentrations of STZ-induced diabetic rats are described in Fig. 3 The serum protein levels were lowered as a result of the diabetic state of the experimental rats. The decreases in the STZ-induced diabetic groups (Groups 2 to 5) are 0.75, 0.95, 0.99 and 1.46 mg/dL. However, gradual increases in serum protein were observed during the 21-day test both for the rats administered PEME of
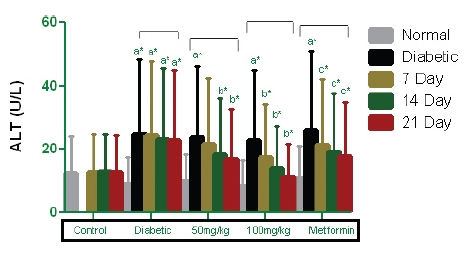
The analyses of the biochemical parameter revealed an elevation of the serum marker enzyme in the STZ-induced diabetic groups, indicating significant increases in ALT levels. Fig. 4 shows that the PEME-treated groups had lower ALT levels (4.12, 10.23, and 13.47 U/L in the 50-mg/ kg body weight group, and 10.79, 17.73, and 23.46 U/L in the 100-mg/kg body weight group on days 7, 14, and 21, respectively), as did the metformin-treated group (8.98, 13.47, and 16.13 U/L). Significant decreases (
3.2. Histopathology and scanning electron microscopic
evaluation of liver
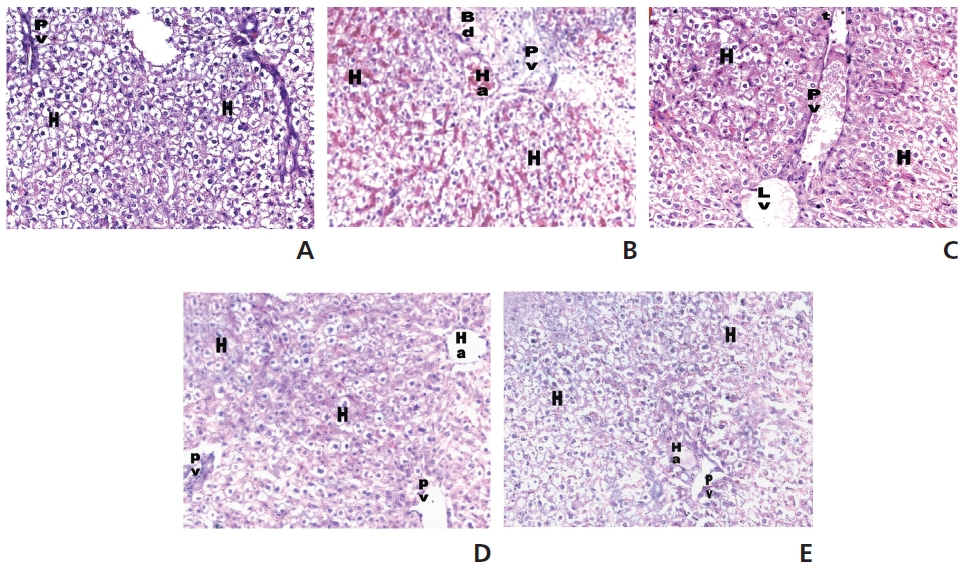
A histological study was carried out by using light microscopy in order to detect whether the PEME treatments with two doses had an effect on the STZ-induced diabetic liver tissues. Pathologically, the liver’s histological structure (Fig. 5A) was normal in the control group (group 1). Fig. 5B shows a liver with complete (severe) destruction of hepatocytes in severe congestion with nuclear condensation, loss of hepatic lobules and congested hepatic inflammation in the diabetic rats. Rats treated with PEME of
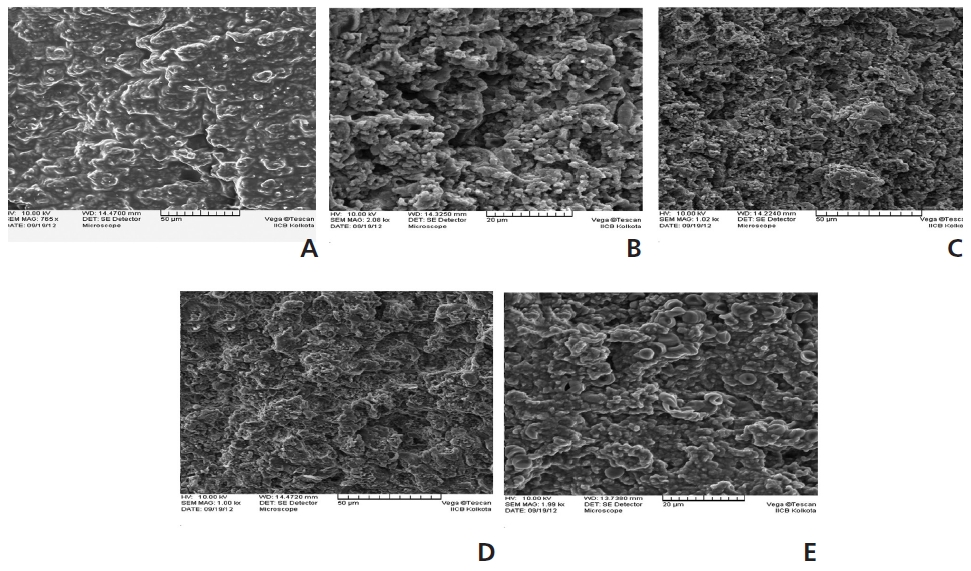
Fig. 6 shows SEM images of the glutaraldehyde control liver, which had not been exposed to STZ. An analysis of the external membrane of a liver from the saline-treated control group showed a regular lobular architecture in the liver membrane and no signs of surface morphological changes, its having a normal structural appearance. Fig. 6B presents SEM images of livers from STZ-induced diabetic rats. These images show marked changes in the surface morphology of the liver. After STZ exposure, the surfaces of the rats’ livers showed shrinkage and appeared to have variously-shaped aggregated tissue granules with a few major pores. External tissue inflammation was also noted in certain areas. Administrations of PEMEs of
STZ is a nitrosurea, a naturally-occurring, broad-spectrum antibiotic, from
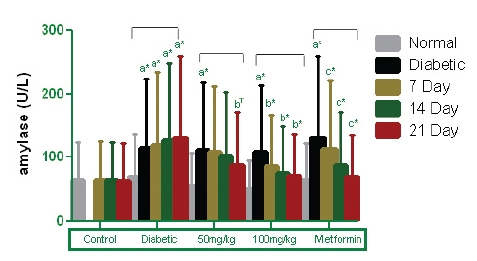
Amylase is a key enzyme in the digestive system that catalyses the hydrolysis of starch to a mixture of smaller oligosaccharides consisting of maltose, maltotriose, and a number of oligoglucans. These are then acted on by α-glucosidases and are further degraded to glucose, which enters the blood stream. The degradation of this dietary starch proceeds rapidly and leads to elevated postprandial hyperglycemia. Inhibitors of
Oxidative stress has been postulated to be an important contributor in diabetes mellitus [14]. During oxidative stress, the reactive oxygen species, which have strong oxidizing ability, damage cellular proteins, causing subsequent changes in serum levels. This action could be due to microproteinuria and increased protein catabolism, which are important clinical markers of diabetes [15, 16]. Another interesting effect of PEMEs of
Hyperglycemia over a long time can cause harmful effects in other tissues, especially in the liver, and liver dysfunction has been seen in diabetic patients with uncontrolled blood sugar levels. Liver function tests conducted through blood assays can provide information about the state of liver damage, describing its cellular integrity and its link with the biliary track [17]. The observed elevated level of alanine transaminase may be indicative of hepatic cholestasis [18] or some other pathological variations in the diabetic conditions. Administrations of PEMEs of
In conclusion, the present study corroborated the beneficial effects of PEMEs of