Recently, extraction methods of natural plant attract attention in the biology and technology on industries. The advantages of using ultrasonication in the extraction of plant compounds have already been demonstrated for various compounds of interest to both the pharmacology and the food industries (Ishtiaq et al. 2009). For example, use of ultrasound in the extraction of tea solids from dried leaves with water has been shown to improve the yield by almost 20% at 60℃, which is almost similar to the efficiency of thermal extraction at 100℃. The main areas of focus for improving sonication methods for extractions is increasing yield and shortening the extraction time (Salisova et al. 1997, Valachovic et al. 2001). Ultrasounds produce cell disruption, particle size reduction, and an ultrasonic jet toward the solid’s surfaces, thereby increasing the area of contact between the solid and liquid phases, which provides better access of the solvent to the valuable components (Mason and Cordemans 1996). Currently, sonication is also employed to extract active compounds such as rutin and quercetin (Yang and Zhang 2008), antioxidants (Albu et al. 2004), polysaccharides (Yang et al. 2007), and bioactive principles (Vinatoru et al. 1997) from plant materials. However, until date, no such method has been reported for the extraction of dried
Brown seaweed
>
Preparation of dried Ecklonia cava
The
>
Preparation of Ecklonia cava extracts by ultrasonic extraction (UE)
An ultrasonic bath was used as the ultrasound source. The bath (JAC 2010; Kodo Technical Research Co. Ltd., Hwaseong, Korea) is a rectangular container (30 cm × 24 cm × 15 cm), with 40-kHz transducers annealed to its bottom; the bath power rating is 200 W. The extraction of
>
Preparation of Ecklonia cava extracts by conventional extraction (CE)
The ground
>
Measurement of the extraction yield
The yields of
>
Determination of the total polyphenolic content
The phenolic contents were determined by using a protocol similar to that used by Chandler and Dodds (1983) and described by Shetty et al. (1995). A mixture of 1 mL of the
>
Radical-scavenging assay using an electron spin resonance (ESR) spectrometer
The DPPH radical-scavenging activity was measured using the method described by Nanjo et al. (1996). The methanol solution of 60 μL of each sample (or only methanol as control) was added to 60 μL of DPPH (60 μmol L-1) in methanol. After mixing vigorously for 10 s, the solutions were transferred into a 100-μL Teflon capillary tube and fitted into the cavity of ESR spectrometer (JES-FA machine; JEOL, Tokyo, Japan). The spin adduct was measured on the ESR spectrometer after exactly 2 min. Measurement conditions: central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 5 mW, gain 6.3 × 105, and temperature 298 K.
Hydroxyl radicals generated by Fenton reaction reacted rapidly with nitrone spin trap DMPO, and the resultant DMPO-OH adducts was detectable on the ESR spectrometer. The ESR spectrum was recorded 2.5 min after mixing phosphate-buffered saline (PBS, pH 7.4) with 20 μL of 0.3 M DMPO, 20 μL of 10 mM FeSO4, using the ESR spectrometer set at the following conditions: central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 5 mW, gain 6.3 × 105, and temperature 298 K.
Alkyl radicals were generated by AAPH. The PBS reaction mixtures, which contained 40 mmol L-1 AAPH, 40 mmol L-1 4-POBN, and the indicated concentrations of tested samples, were incubated at 37℃ in a water bath for 30 min (Hiramoto et al. 1993) and then transferred into a 100-μL Teflon capillary tube. The spin adduct was recorded on the ESR spectrometer set at central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 5 mW, gain 6.3 × 105, and temperature 298 K.
>
Hydrogen peroxide (H2O2)-scavenging assay
The H2O2-scavenging activity was determined according to the method of Muller (1985). Then, 100 μL of 0.1 M phosphate buffer (pH 5.0) was mixed with the sample solution in a 96-well plate. To this mixture, 20 μL of H2O2 was added and incubated at 37℃ for 5 min. Next, 30 μL of 1.25 mM ABTS and 30 μL of peroxidase (1 unit mL-1) were added to the mixture and incubated at 37℃ for 10 min. The absorbance was read with an enzyme-linked immunosorbent assay reader at 405 nm.
To study the inhibition effect of the extracts against H2O2-mediated DNA damage, we used the L5178 mouse T-cell lymphoma cell line (L5178Y-R). This cell line was maintained at 37℃ in an incubator under a humidified atmosphere of 5% CO2 and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), streptomycin (100 μg mL-1), and penicillin (100 unit mL-1).
The alkaline comet assay was conducted according to the method described by Ahn et al. (2007). The number of cultured cells was adjusted to 4 × 104 cells mL-1, and the cells were incubated with each samples (concentrations ranging from 25 to 100 μg mL-1), as determined according to the H2O2-scavenging activity for 30 min at 37℃ in dark. The cells were then centrifuged at minimum speed for 5 min and washed using PBS. The cells were resuspended in PBS with 50 μM H2O2 for 5 min on ice. The untreated control cells were resuspended in PBS only. Then, the cells were washed with 1 mL PBS and centrifuged. The cell suspension was mixed with 100 μL of 0.7% low melting point agarose (LMPA) and then added to 1.0% normal melting point agarose-coated slides for 10 min at 4℃. The slides were then covered with another 100 μL of 0.7% LMPA and kept for 40 min at 4℃ for the solidification of agarose. Next, the slides were immersed in the lysis solution (2.5 M NaCl, 100 μM EDTA, 10 mM Tris, 1% sodium lauryl sarcosine, and 1% Triton X-100) for 1 h at 4℃. The slides were unwinded and applied for electrophoresis with the electric current of 25 V/300 mA for 20 min. Then, the slides were neutralized in 0.4 M Tris buffer (pH 7.5) for 10 min, two times, and dehydrated with 70% ethanol. The percentage of fluorescence in the DNA tail of each cell (tail intensity, TI; 50 cells from each of two replicate slides) on ethidium bromide-stained slides was measured by image analysis (Komet 5.0; Kinetic Imaging, Liverpool, UK) and under fluorescence microscope (DMLB; Leica, Wetzlar, Germany).
Data were analyzed using the SPSS version 10 (SPSS Inc., Chicago, IL, USA). The values were expressed as mean ± standard error (SE). The mean values of the tail intensity from each treatment were compared using oneway analysis of variance (ANOVA), followed by Duncan’s multiple range test. p-Value of less than 0.05 was considered significant.
>
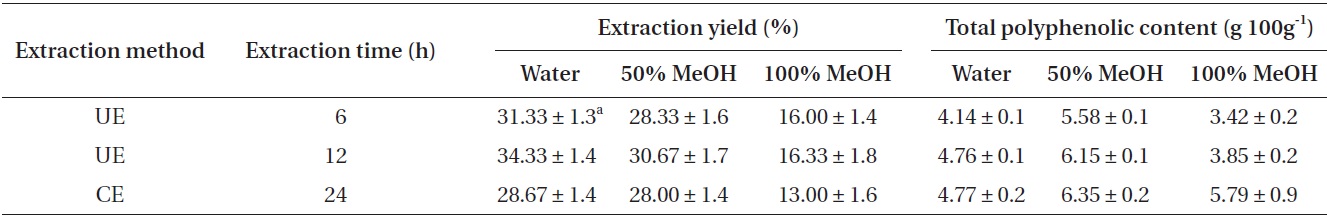
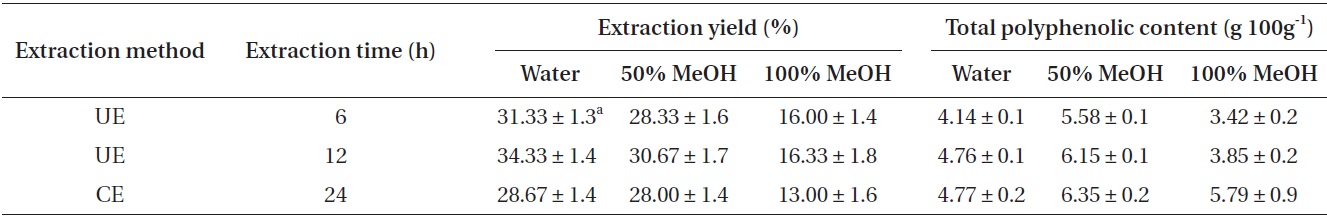
Extraction yield and total polyphenolic contents
The extraction yield and total polyphenolic content of extracts obtained by UE and CE from
The highest polyphenolic content (6.35 g 100 g-1) was detected in the 50% methanolic extract prepared using CE 24 h, whereas the lowest content (3.42 g 100 g-1) was detected in the 100% methanolic extract prepared using UE 6 h. In the case of UE, the total polyphenolic content increased as the UE time increased. The 50% methanolic extracts showed higher total polyphenolic content than the water extracts, and, all the extracts treated by CE for 24 h exhibited higher total polyphenolic content than UE treated for 6 and 12 h (Table 1).
>
Reactive oxygen species (ROS)-scavenging activity
In this study, the ROS-scavenging activities of all
Extraction yields and total polyphenolic contents as affected by different extraction methods
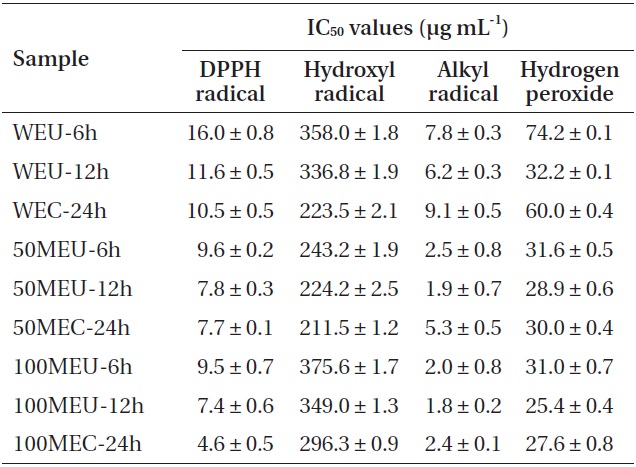
CE showed the highest DPPH radical-scavenging activity at IC50 of 4.6 μg mL-1. Under similar condition, the 100% methanolic extracts exhibited higher DPPH radical-scavenging activity than other extracts. Hydroxyl radicals generated in the Fenton system (Fe2+/H2O2) were trapped by DMPO to form a spin adduct, as detected by the ESR spectrometer. All of the tested extracts showed good hydroxyl radical-scavenging activity (Table 2). From among them, the 50% methanolic extract prepared by CE exhibited the highest scavenging activity (IC50, 224.2 μg mL-1). In case of UE, the hydroxyl radical-scavenging activity increased as the UE time increased. However, all extracts prepared by UE showed lower hydroxyl radical-scavenging activity than those prepared by CE. The alkyl radical spin adduct was observed when AAPH was incubated with the spin trap 4-POBN at 37℃ for 30 min. All of the tested extracts showed strong alkyl radical-scavenging activity. In case of UE, alkyl radical-scavenging activity increased as the UE time increased. Moreover, all the extracts prepared by UE showed higher alkyl radical-scavenging activity than those prepared by CE did. Among them, the 100% methanolic extract prepared by UE 12 h exhibited the highest scavenging activity at IC50 of 1.8 μg mL-1. All the extracts showed good H2O2-scavenging activity. Among them, the 100% methanolic extract prepared using UE 12 h exhibited the highest scavenging activity at IC50 of 25.4 μg mL-1 (Table 2). Under the same condition, the 100% methanolic extracts showed higher H2O2-scavenging activity than did other extracts. In case of UE, the H2O2-scavenging activity increased as the UE time increased. Moreover, all extracts prepared by UE 12 h showed higher H2O2-scavenging activity than extracts prepared by CE. On the other hand, the extracts prepared by UE 6 h showed lower H2O2-scavenging activity than extracts prepared by CE. Thus, from among all tested extracts for H2O2-scavenging activity, 100% methanolic extract with UE 12 h (100MEU-12h) and 100% methanolic extract with CE 24 h (100MEC-24h) were selected and evaluated by comet assay for their inhibitory effect against H2O2-induced DNA damage.
[Table 2.] Radical scavenging properties (IC50 values) of different extracts of Ecklonia cava
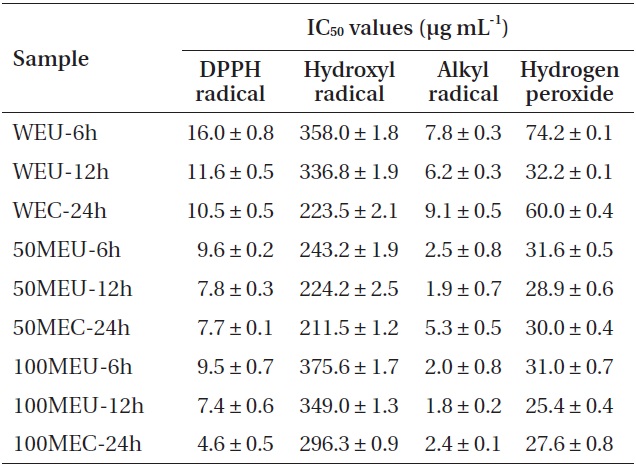
Radical scavenging properties (IC50 values) of different extracts of Ecklonia cava
>
Inhibitory effect of DNA damage of H2O2-induced cells
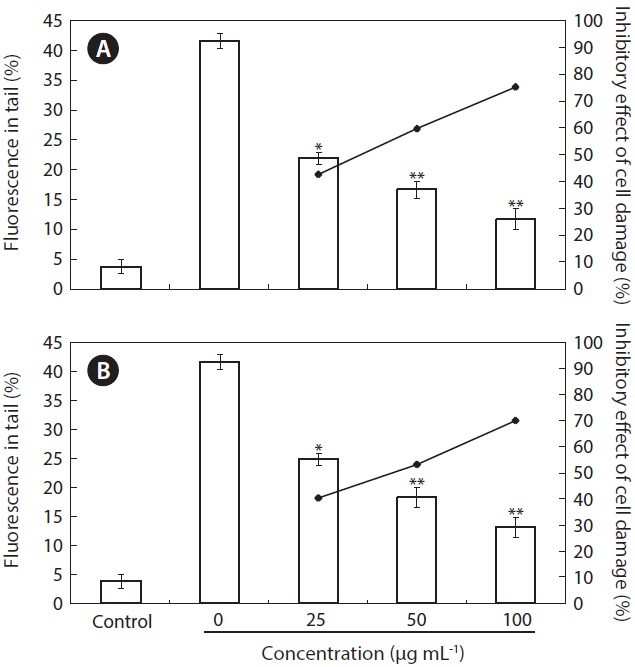
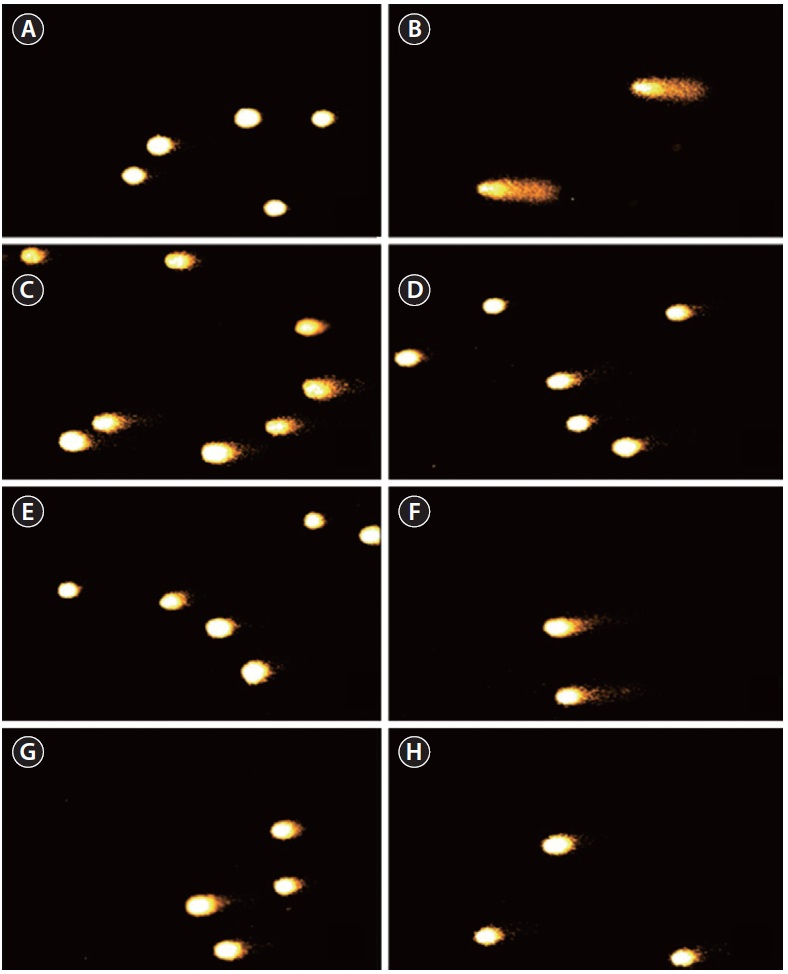
The inhibitory effect of 100MEU-12h and 100MEC-24h against H2O2-induced DNA damage was investigated by using comet assay (Figs 1 & 2). The percent fluorescence in DNA tail intensity of L5178 cells was significantly increased in cells treated with only H2O2. The level of DNA damage induced by H2O2 was significantly controlled dose-dependently by pre-incubating H2O2 with 100MEU-12h and 100MEC-24h at the concentrations of 25, 50, and 100 μg mL-1 in PBS (Fig. 1). At the same concentrations, 100MEU-12h exhibited slightly higher protective effect against H2O2-induced DNA damage than 100MEC-24h. Fig. 2 shows the photomicrographs of different DNA migration profiles obtained from L5178 cells in different concentrations of 100MEU-12h (Fig. 2C-E) and 100MEC-24h (Fig. 2F-H). In the cells exposed to only H2O2, the DNA was completely damaged. However, the addition of 100MEU-12h and 100MEC-24h with H2O2 effectively suppressed the DNA damage; in particular, 100MEU-12h at 100 μg mL-1 showed highest inhibitory effect of DNA damage.
Bioactive compounds such as polyphenols, pigment, fucoxanthin, terpenoids, and polysaccharide are found in various seaweeds. For this reason, there is a growing interest in extraction these seaweed bioactive compounds and using them as functional materials. Among them,
The CE extraction method involves consumption of large amount of solvent and a long extraction time (Yan et al. 1999). Extraction of bio-compounds from plants is generally performed using conventional methods. However, other techniques, including supercritical carbon dioxide extraction, subcritical water extraction, UE, and microwave extraction have also gained interest as alternatives to the conventional methods (Wang and Weller 2006). Among these, the UE method offers substantial advantages for the extraction of organic compounds from plants and seeds using a solvent. The mechanical effects of ultrasound provide a greater penetration of solvent into the cellular materials and improve mass transfer (Mason and Cordemans 1996). In addition, the use
of power ultrasound in extraction results in the disruption of biological cell walls, which facilitate the release of contents. Previous studies reported that advantages UE technology can reduces extraction time and solvent use (Mason et al. 1996). Thus, in the present study, the water and the methanolic extracts of
The results showed that the extraction yield and total polyphenolic content increased as the UE time increased. All the UE extracts exhibited higher extraction yield than CE extracts. The water extract by UE treated for 12 h showed 6% improvement in yield compared with CE extracts treated for 24 h. On the other hand, in case of total polyphenolic content, all the CE extracts exhibited slightly higher total polyphenolic content than UE extracts. The results indicate that the extraction yield and total polyphenol content are highly time-dependent. A previous study has reported that, in almost all cases of extraction by UE, the amount of extract is similar or greater as compared with that in the conventional techniques (Sun et al. 2013). The ultrasonic procedure thus seems to be a significant improvement in terms of the extraction time (Vinatoru et al. 1997).
Free radicals or ROS create oxidative stress, which leads to a variety of physiological lesions and often results in metabolic impairment such as inflammation, aging, cancer, and hypertension. In this study, the free radical-scavenging activities were evaluated by ESR technique. Spin trapping ESR was the most direct method to detect highly reactive free radicals generated for short times (Janzen et al. 1987). DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule, and has often been used as a substrate to evaluate the antioxidative activity of natural compounds (Soares et al. 1997). Hydroxyl radicals are the major ROS causing enormous biological damage and the initiation of lipid peroxidation. Hydroxyl radicals generated in the Fenton system (Fe2+/H2O2) was trapped by DMPO, forming a spin adduct as detected by the ESR spectrometer. The alkyl radical has been found to be a primary intermediate in many hydrocarbon reactions. These radicals are easily detected with ESR, which has been found to be very useful in the characterization of solid surfaces, and in the elucidation of active surfaces sites as well as surface reactions (Adebajo and Gesser 2001).
The antioxidative effects of all extracts by UE and CE from
DNA damage can destroy cells and organism and generate various diseases in human. We used the comet assay in this study and it is a sensitive, direct and accurate method and can measure inhibitory activities on H2O2-induced DNA damage. As 100MEU-12h and 100MEC-24h from
The antioxidative effects on radical-scavenging and H2O2-induced DNA damage in the far infrared radiation dried