Depression is a common mental disorder and one of the most important causes of disability in the world, with a heavy social burden and a substantial lifetime risk1). Depression is frequently recurrent and chronic, and it has been associated with suicide risk and psychosocial dysfunction2). In the United States, lifetime prevalence is about 10∼ 20%3). The lifetime suicide attempt rate is greater than 15% in patients suffering from major depression 4).
Antidepressant therapy includes drugs with exceptional structural chemical diversity; most of them increase monoaminergic neurotransmission5). Although the majority of the antidepressant drugs ameliorate depressive symptoms, they exert multiple unwanted side effects; moreover, 30% of depressive patients do not react appropriately to the first-line treatment6). Moreover, a major limitation of available monoaminergic-based antidepressant drugs is their lag period for therapeutic effects, which often approaches several weeks. Indeed, the lag period in onset of action of traditional antidepressants is resulting in considerable morbidity and high risk of suicidal behavior especially in the first 9 days after starting antidepressant treatment7). Thus, the search for more efficacious and well-tolerated drugs is in progress, and this has led to an interest in glutamatergic-based potential therapeutics 8-10).
The dysregulation of glutamatergic systems has been implicated in various stages of psychiatric disorders. Abnormalities in glutamatergic systems have been consistently identified in major depressive disorder (MDD)11-14). For example, the glutamate levels in the occipital cortex are abnormally elevated in MDD patients15). In contrast, a reduction in Glx levels (a marker for combined glutamate and glutamine) has been observed in the anterior cingulate cortex of patients with MDD16). A similar reduction was also reported in the anterior cingulated cortex in children with major depression17).
Accumulated evidence demonstrated that Nmethyl- D-aspartate (NMDA) receptor ligands (functional antagonists) interacting with different components of the NMDA receptorionophore complex produced antidepressant-like effects. The preclinical experiments showed that noncompetitive (MK-801) and competitive antagonists (CGP37849), as well as antagonists (L-701,324) and partial agonists of the glycine/NMDA site (D-cycloserine) produced antidepressant-like effects in the forced swim test (FST)18-22). Furthermore, ketamine (a noncompetitive antagonist of the NMDA receptor complex) and CP-101,606 (a selective antagonist of NR2B subunit of NMDA receptor) is effective in human depression23-25). In randomized, placebo-controlled, double blind study, robust and rapid antidepressant effects resulted from a single intravenous dose of ketamine. Onset occurred within 2 hours postinfusion and continued to remain significant for 1 week24).
The role of
The need for the discovery and development of new pharmaceuticals for the treatment of depression demands that all approaches to drug discovery be exploited. Among the possible approaches, the use of natural products has made many unique and vital contributions to drug discovery31). In this regard, many medicinal plants have been used as a treatment for stress, sadness, anxiety and depression 32). In this regard, Traditional Korean Medicine (TKM) may possess a high potential for treating depression.
Gastrodiae Rhizoma is the dried tuber of Gastrodia elata Bl. (GE) which belongs to the Orchidaceae family and is widely-used in many Asian countries, including Korea, China, Taiwan, Japan, and others, as a traditional medicine for the treatment of many diseases. However, GE is difficult to cultivate, it grows in the forest at 400∼3,200 meters above sea level in the temperate zone. It is a myco-heterophyte that has a symbiotic relationship with the fungus Mycenaosmundicola for germination and Armillaria mellea fornutrition on rotten wood33-35). In Chinese, GE is known as chi jian, gui d you, or tin ma. GE is a famous traditional medicine used for centuries. GE is recorded in many traditional medicine classics. According to these classics36-39), GE is a top grade medicine that enters the liver channel (gn jng) and is used to treat ailments such as headache, dizziness, convulsion, paralysis, rheumatism, and lumbago. In summary, GE enhances health and prolongs life. Recently, the biological activity and the active compounds of GE are popular study topics by researchers worldwide, although most studies mainly come from Korea, China, Taiwan and Japan. There are many scientific evidences supporting the biological activities of GE. GE treatment exerts an effective inhibition of diverse diseases and disorders, including convulsion 40-43), oxidative stress41,44,45), mental disorders 46-49).
The effect of GE on mental disorders such as depression, anxiety, and schizophrenia has been investigated by animal models. The antidepressant effect of GE was evaluated in animal models and several mechanisms of activity were found. Accumulated evidence demonstrated that N-methyl- D-aspartate (NMDA) receptor ligands (functional antagonists) interacting with different components of the NMDA receptorionophore complex produced antidepressant-like effects. A current focus of schizophrenia research is on two major brain neurotransmitters, dopamine (DA) and glutamate, both of which may be altered in schizophrenia. NMDA receptor is important one of glutamatergic system. So, anti-psychotic effect of GE on schizophrenia in a recent study50), imply that GE acts possibly on glutamate system, but, direct interaction with glutamate receptors is still unclear.
In view of these findings, this study was performed to investigate the antidepressant-like effects of ethanolic extract of GE, in behavioral despair models examined using FST and TST, both of which are commonly used tests for assessing antidepressant activity51-53). Locomotor activity was monitored for 10 min in an open field. Additional behavioral experiments were undertaken to determine whether the antidepressant-like properties of GE involve AMPA receptor throughput.
Male C57Bl/6 mice (ORIENT BIO Inc., Korea) weighing 2,022 g, were used for behavioral and biochemical experiments. Animals were housed in acrylic cages (22×27×12 cm) with free access to water and food under an artificial 12-h light/dark cycle (lights on/off at 7:00 A.M/P.M.) and at a constant temperature (22±2℃). Mice were housed in the departmental room for 1 week before testing to ensure adaptation to the new environment. All of the behavioral experiments were performed between 9:00 and 15:00. This study was performed in accordance with the Kyung-Hee University Laboratory Animal Research Center guidelines for the care and use of laboratory animals.
GE was purchased from an oriental drug store (Muju Chunma Cluster Agency, Korea), collected from Korea (Muju, Jeollabukdo, Korea) in September, 2010. The dried GE samples (856 g) were immersed in 40% ethanol (4,300 ml), boiled at 80℃ for 6 h, and then the 40% ethanolic extract was collected. The extracts were filtered through a filter paper. The filtrate was evaporated on a rotary evaporator under reduced pressure and temperature, and freezing-dried to yield about 50% (w/w) of the extract. The crude extracts were completely solubilized in distilled water (DW) for use in this experiments, and administerted to the mice orally (100, 400, 1,600 mg/kg, p.o.). For each drug treatment control mice received the respective vehicle alone.
The FST was carried out according to widely used procedures54). The apparatus consisted of a transparent Plexiglas cylinder (height 35 cm, width 20 cm) filled to a 20 cm depth with water at room temperature. Mice were placed in a cylinder of water between 23 and 25℃. The duration of immobility during the last 4 min of the 6-min test were scored. Mobility was defined as any movement beyond what is necessary to maintain the head above water. At the end of the trial session, mice were taken out of the water, dried with a paper towel and placed back in their home cages. To investigate dose-dependent antidepressant-like effects of 40% ethanolic extract of GE, GE extracts (100, 400, 1,600 mg/kg) or DW were administered p.o. 1 h before the FST. Seven to eight mice were used for each group.
The TST was carried out according to the method described by Steru et al.53), with modifications. Mice were suspended by the tail from a metal rod using adhesive tape. The rod was fixed 45 cm above the surface of a table in a sound-isolated room. The mouse was positioned at least 15 cm away from the nearest object. The test sessions were recorded for 6 min, and the immobility time was determined by an observer. Mobility was defined as movement of the hind legs. To investigate dose-dependent antidepressant-like effects of 40% ethanolic extract of GE, GE extracts (100, 400, 1,600 mg/kg) or DW were administered p.o. 1 h before the TST. Seven to eight mice were used for each group.
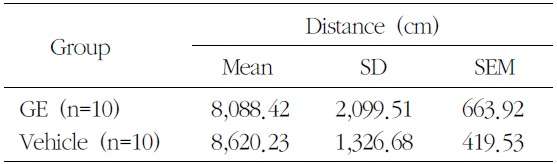
The open-field test was performed after a single dose of vehicle, GE (1,500 mg/kg). In accordance with previous methods55), the apparatus consisted of a square box with dimensions, 50×50×50 cm. Mice were placed into the center of the open box under a dark light (25 lx), and allowed to explore the arena for 30 minutes, between the hours of 11:00 a.m 16:00 p.m. A video-computerized tracking system (SMART, Panlab SL, Barcelona, Spain) was used to record the distance traveled, as a measure of locomotor activity.
Statistical analyses were performed using computer software (PASW Statistics 18; SPSS Inc., Chicago, IL). Data were analyzed using one-way analysis of variance (ANOVA) and independent t-test. Tukey HSD post hoc test and LSD post hoc test were utilized to compare significant ANOVA results. Results are presented as the mean±SEM. All statistics were 2-tailed. A p-value less than 0.05 is considered significant.
We examined the effects of GE on depression- like behavior, forced swimming test and tail
[Table 1.] Effects of the Ethanolic Extranct of GE on Open Field Test
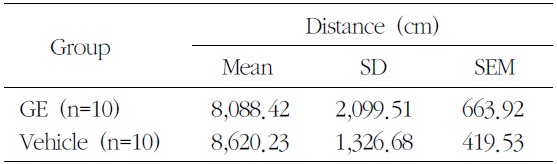
Effects of the Ethanolic Extranct of GE on Open Field Test
suspension test. Locomotor activity was monitored for 10 min in an open field. In the open field test, there was no difference (p=0.507) between GE and vehicle (Table 1).
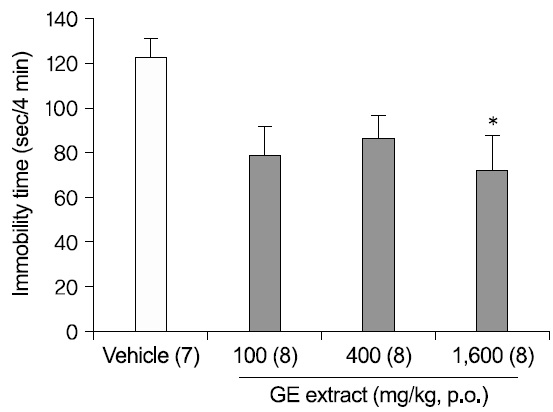
1. Antidepressant-like effects of GE extract in the FST
To investigate dose-dependent antidepressantlike effects of 40% ethanolic GE extract, the FST was used as described in Methods. Mice were treated with DW or GE extract (100, 400, 1,600 mg/kg), 1 h before the test (Fig. 1). An oral administration of GE extract 1,600 mg/kg significantly reduced the immobility times in the FST [F(3,27)=3.101; p=.043; DW vs. GE 1,600 mg/kg p=.041], though GE extract 100 mg/kg and 400 mg/kg didn't significantly reduced the immobility times.
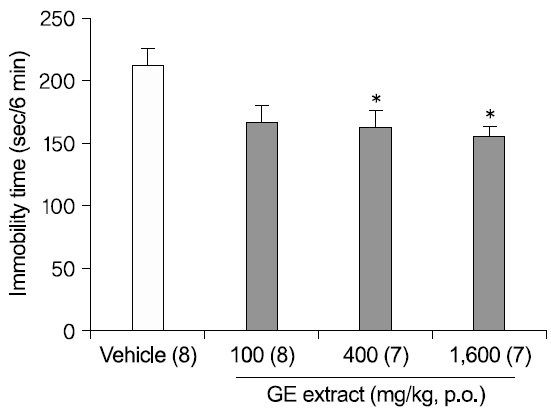
2. Antidepressant-like effects of GE extract in the TST
We were interested in determining whether an oral administration of GE extract had antidepressant- like effects in another validated mouse model of antidepressant efficacy, the TST. As described in Methods, mice were treated with DW or GE extract (100, 400, 1,600 mg/kg), 1 h before the test (Fig. 2). An oral administration of GE extract 400 mg/kg and 1,600 mg/kg significantly reduced the immobility times in the TST [F(3,26)=4.373; p=.013; DW vs. GE 400 mg/kg p=.048; DW vs. GE 1,600 mg/kg p=.017], though GE extract 100 mg/kg didn't significantly reduced the immobility times (p=.056).
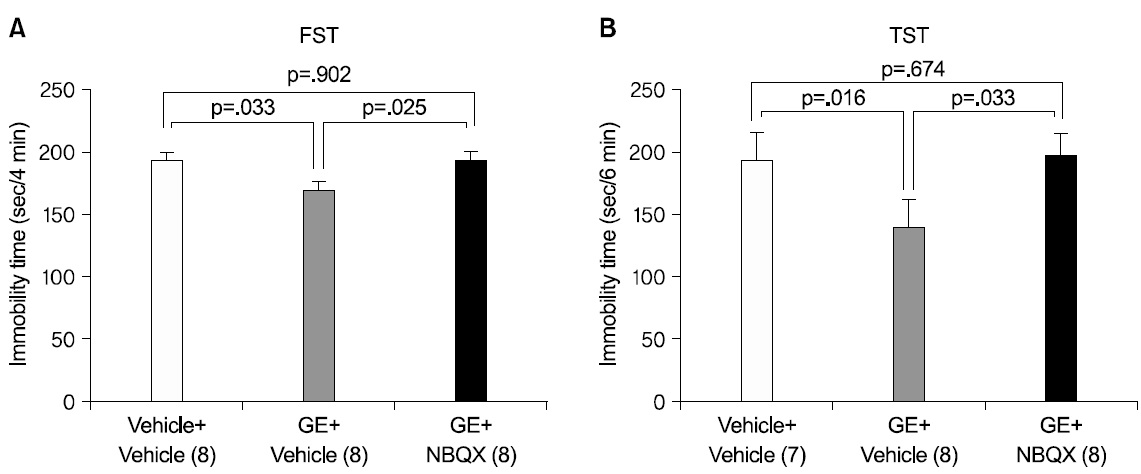
3. Effects of AMPA receptor blockade on the antidepressant-like effects of GE extract
We hypothesized that regulation of AMPA receptor activation may be implicated in the antidepressant- like effects of 40% ethanolic extract of GE in the FST and TST. To determine whether activation of AMPA receptors is required for the antidepressant- like effects of GE extract in the FST and TST, we administered GE extract 1,600 mg/kg or DW to mice 1 h before the test, followed by administration of NBQX (an AMPA receptor antagonist) or vehicle 40 minutes prior to testing in these behavioral tasks. We chose a dose of 10 mg/kg because a dose in this range has been shown to decrease AMPA-induced seizures in mice56). NBQX pretreatment significantly attenuated the reduction in immobility time induced by GE extract in the FST (Fig. 3A) [F(2,21)=3.690; p=.042]. Post hoc analysis revealed a significant effect of GE extract alone compared to the each of the other 2 groups (both, p<.05) and no other significant differences. Similarly, the antidepressant-like effects of GE extract 1,600 mg/kg administration in the TST were attenuated by NBQX (Fig. 3B) [F(2,20)=4.157; p=.031]. Post hoc analysis revealed a significant effect of GE extract alone compared to each of the other 2 groups (both, p<.05), and no other significant differences. We omitted the group treated by DW and NBQX, because, for both the FST and TST there was no significant effect of NBQX alone to change immobility time compared to vehicle treated mice in previous studies28,30,57,58).
Modulation of the AMPA receptor subclass of glutamate receptors has been linked to the mechanism of action of antidepressant medications as well as a target for future medications11,59-64). It has recently been reported that the antidepressant-like effects of ketamine in the FST, as well as the NMDA antagonist MK801 and NR2B antagonist, Ro25-6981, are prevented by preadministration of NBQX28). But it is unclear what the mechanism is by which modulation of AMPA receptors may be involved in the mechanism of antidepressant action, or to what extent AMPA activation is required for therapeutic action in humans26).
A major limitation of available monoaminergicbased antidepressant drugs is their lag period for therapeutic effects, which often approaches several weeks. Indeed, the lag period in onset of action of traditional antidepressants is resulting in considerable morbidity and high risk of suicidal behavior especially in the first 9 days after starting antidepressant treatment7). Accumulated evidence demonstrated that N-methyl-D-aspartate (NMDA) receptor antagonists produced rapid antidepressant- like effects. A current focus of schizophrenia research is on two major brain neurotransmitters, dopamine (DA) and glutamate. NMDA receptor is important one of glutamatergic systems. So, anti- psychotic effect of GE on schizophrenia in a recent study50), imply that GE acts possibly on glutamate system.
A paradigm shift from a monoamine hypothesis of depression to a neuroplasticity hypothesis focused on glutamate may represent a substantial advancement in the working hypothesis that drives research for new drugs and therapies. BDNF is confirmed to be involved with depression65), as it is decreased in depressed patients66) and is elevated upon treatment with antidepressants65,67,68). Therefore, elevation of BDNF might be one of the antidepressant mechanisms used by GE, as it has been shown to increase the expression of BDNF genes in an animal model69).
The effect of GE on mental disorders such as depression, anxiety, and schizophrenia has been investigated by animal models. In both the forced-swimming test and tail-suspension test, animal models used for evaluation of antidepressant activity, the water and the ethanol extract of GE demonstrated a significant antidepressant effect70-72). Both the serotonergic and the dopaminergic systems in the rats' brain were significantly increased by the water extract of GE. In this model, the concentrations of serotonin and dopamine were elevated, while the turnover of these two monoamines was decreased after orally GE treatment71). Furthermore, the water extract of GE attenuated memory impairment loss by the animals as measured by the inhibitory avoidance task and the Morris water maze in rats73). This is a significant finding, as memory impairment is a common problem that affects patients with depression. The water extract of GE, containing the 4-hydroxybenzyl alcohol and 4-hyroxybenzaldehyde active compounds, showed anxiolytic effects in the elevated plus-maze test in mice. This study further showed that the anxiolytic effects of these compounds might involve the serotonergic or GABAergic system74).
The antidepressant effect of GE has been supported by various animal studies. However, the mechanisms behind its antidepressant activity need further investigation. In animal models of depression, studies suggest that GE mediates its antidepressant activity by modulating monoamineoxidase enzyme activity as well as monoamine concentration and turnover. Indirect evidence showed that antioxidant activity, GABAergic regulation, BDNF modulation, neuroprotection, and anti-inflammatory effects might also contribute to the antidepressant effect of GE75). Furthermore, this study suggests that the antidepressant effect of GE is related with AMPA receptor activation.
In the present study, the ehthanolic fractions of GE were devoid of qualitative content, therefore, this finding warrants further study in order to establish which constituents contribute to the antidepressant- like effects of GE extract. Additional animal studies (such as Learned helplessness paradigm) are also necessary to demonstrate the rapid and sustained antidepressant-like effects of GE extract. To clarify the effect of GE on glutamatergic systems, further well-designed study is needed. Further characterization of these action of constituents of GE and the signaling pathway that activate AMPA receptor will provide novel therapeutic agents for antidepressant drug development.
This study was performed to investigate the antidepressant- like effects of 40% ethanolic extract of GE, in behavioral despair models examined using FST and TST. In addition, behavioral experiments were undertaken to determine whether the antidepressant- like properties of GE involve AMPA receptor throughput. The results of this study were as follows.
1. An oral administration (1 h before the test) of GE extract 1,600 mg/kg significantly reduced the immobility times in the FST (p=.040).
2. An oral administration (1 h before the test) of GE extract 400 mg/kg or 1,600 mg/kg significantly reduced the immobility times in the TST (p=.048; p=.017).
3. NBQX pretreatment (10 mg/kg, 40 min before the test, i.p.) significantly attenuated the reduction in immobility time induced by GE extract (1,600 mg/kg, 1 h before the test, p.o.) in the FST and TST. Post hoc analysis revealed a significant effect of GE extract alone compared to the each of the other 2 groups (both, p<.05), and no other significant differences.
In conclusion, ethanolic extract of GE might exert antidepressant-like effects in relation to AMPA receptor.