Surimi is defined as the deboned, water-washed, and minced fish meat with cryoprotectives such as sorbitol, sucrose and polyphosphates (Khan et al., 2003; Hunt et al., 2009). A mixture of fish meat protein and salt forms a three-dimensional protein network during the heating process due to crosslinking by the denaturation of protein molecules (Powrie and Tung, 1976; Kong et al., 1999a, 1999b). The rheological properties of surimi gel depend on the fish species, fish quality, salt content, additives, and processing methods (Okada, 1981; Kong et al., 1999a, 1999b). To better address the textural properties of surimi-based products for consumers, additives such as starch, beef plasma protein, gum, egg white, and soy protein have been used (Khan et al., 2003; Jafarpour et al., 2012).
Carrageenan has also been used as a gelatinizing additive to enhance the texture and water-holding capacity of water-based gel systems, dairy products, meat and poultry products, and seafood systems (Hunt and Park, 2013). Carrageenan is a sulfated and linear polysaccharide with a chemical structure consisting of repeating units of galactose and 3,6-anhydrogalactose (Trius and Sebranek 1996; Chiovitti et al., 1997; Hunt and Park, 2013). There are three forms of carrageenan, kappa (κ), lambda (ι), and iota (λ), and their gelling abilities are known to be affected by cations such as K+, Na+, and Ca2+ (Ortiz and Aguilera, 2004). The gelling ability of κ-carrageenan in aqueous solution is enhanced by K+, resulting in increased utility of κ-carrageenan in K+-induced products (Abbasi and Dickinson, 2004; Ortiz and Aguilera, 2004).
Several studies have examined the interactions of refi ned carrageenan with fish protein and the effect of different types of cations in a fish protein-carrageenan system (Montero and Perez-Mateos, 2002; Sarıcoban et al., 2010; Brewer, 2012). However, few studies have investigated the interactions of carrageenan with Alaska pollock meat protein when affected by various salts. Therefore, in the present study we investigated the additional effect of κ-carrageenans on several salt-based surimi gels.
Frozen Alaska pollock surimi
Surimi gel was prepared using frozen surimi with κ-carrageenan, NaCl and KCl. Frozen surimi was tempered in a chilled room, chopped into small pieces of 2 × 2 × 2 cm3, and ground by a refrigerated food cutter (Hanil Co. Ltd., Seoul, Korea) for 2 min at high speed. The different types of salt, 2% (w/w) NaCl, 1.5% (w/w) KCl, and their mixture of 2% (w/w) NaCl and 1.5% (w/w) KCl were added to the ground surimi and homogenized for 3 min at slow speed. Then, a hydrocolloid κ-carrageenan at a concentration of 0%, 0.2%, or 1.0%, and deionized water, were added
[Table 1.] Hunter color values of 1% κ-carrageenan-added surimi gel as affected salts concentration
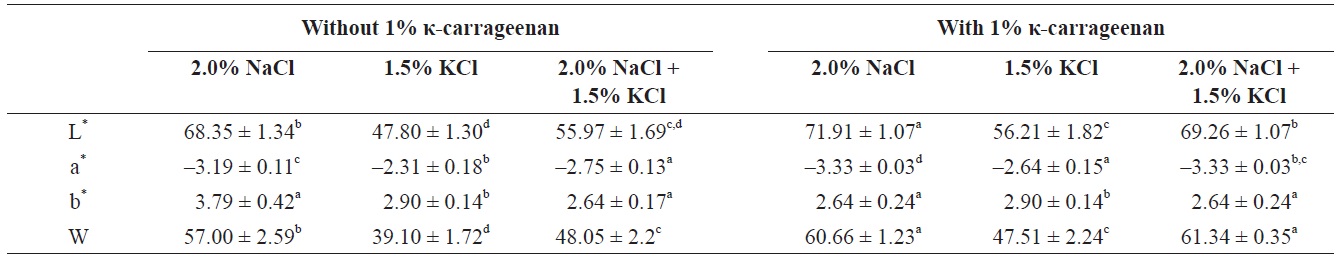
Hunter color values of 1% κ-carrageenan-added surimi gel as affected salts concentration
to each sample, followed by an additional 5 min of grinding at slow speed. The prepared fish meat paste was extruded into a polyvinylidene chloride casing. The stuffed casing was heated in a hot-water bath (HB-205WP; Hanbaek Scientific Co., Bucheon, Korea) at 90 ± 2℃ for 30 min. After heating, the surimi gels were immediately cooled in ice water (0-4℃) for 20 min to stop any further action due to heating. The gels were stored overnight at 4℃ before analysis.
The surimi gel was sliced at a thickness of 1.5 cm and color was determined using the Color Difference Meter (Lovibond Tintometer Model RT 300; Tintometer Ltd., Salisbury, UK). The Hunter color parameters such as L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) values were recorded (
Whiteness = (L* ? 3b*).
The prepared surimi gels were cut into small disks, 2.5 cm in height and 2.6 cm in diameter, tempered at 20℃, and subjected to a compression test with a Rheometer (Type COMPAC-100; Sun Science Co., Tokyo, Japan), using a 5-mm diameter cylindrical plunger with up to 9 mm of compressive strain and a compression speed of 60 mm/min. The quality of the gels was assessed by measuring the breaking force (g), deformation (mm), and gel strength (g cm).
The data were expressed as means ± standard deviation (SD). Differences between the means of the individual groups were analyzed by one-way analysis of variance (ANOVA) using the Statistical Analysis System, SPSS version 9.1 (SPSS Inc., Chicago, IL, USA) with Duncan’s multiple range test; statistical significance was defined as
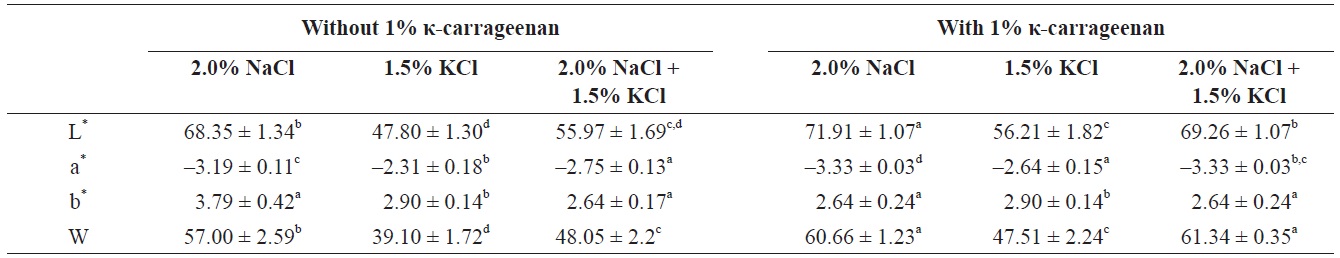
The color parameters of surimi gels with/without κ-carrageenan were compared (Table 1). The color parameters were significantly influenced by the addition of different concentrations of κ-carrageenan. When κ-carrageenan was added, L* values and whiteness of the surimi gels increased. NaClinduced surimi gel with 1% κ-carrageenan showed the highest L* values and whiteness and the lowest a* and b* values. However, the KCl-induced surimi gel showed lower L* values and whiteness than the NaCl-induced surimi gel. In starchadded surimi gels, the changes in a* values were considered to indicate significant changes in myoglobin denaturation (Zhang et al., 2013), while the changes in b* values were considered to be due to the gelatinization and swelling ability of starch (Svihus et al., 2005). Gels became more opaque due to the expanding and swelling of κ-carrageenan granules in the presence of KCl, resulting in decreased gel lightness and whiteness.
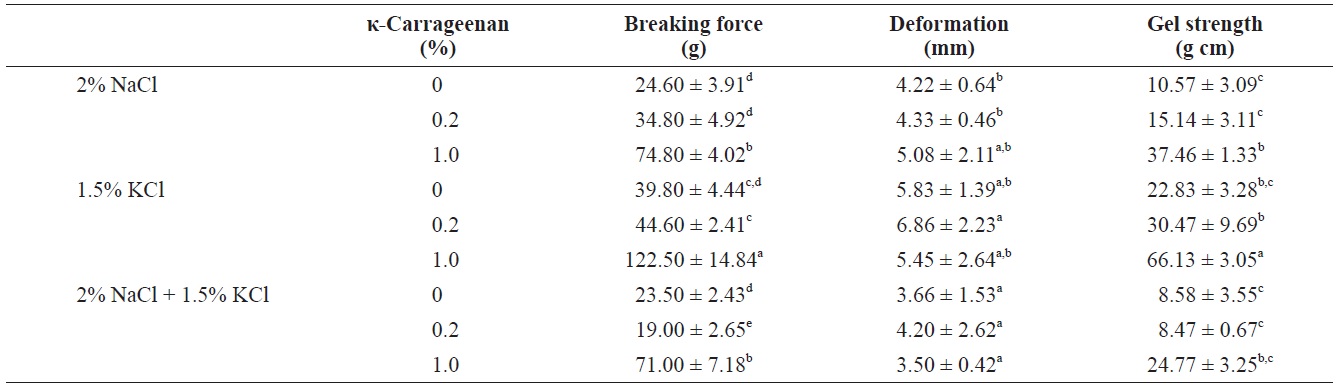
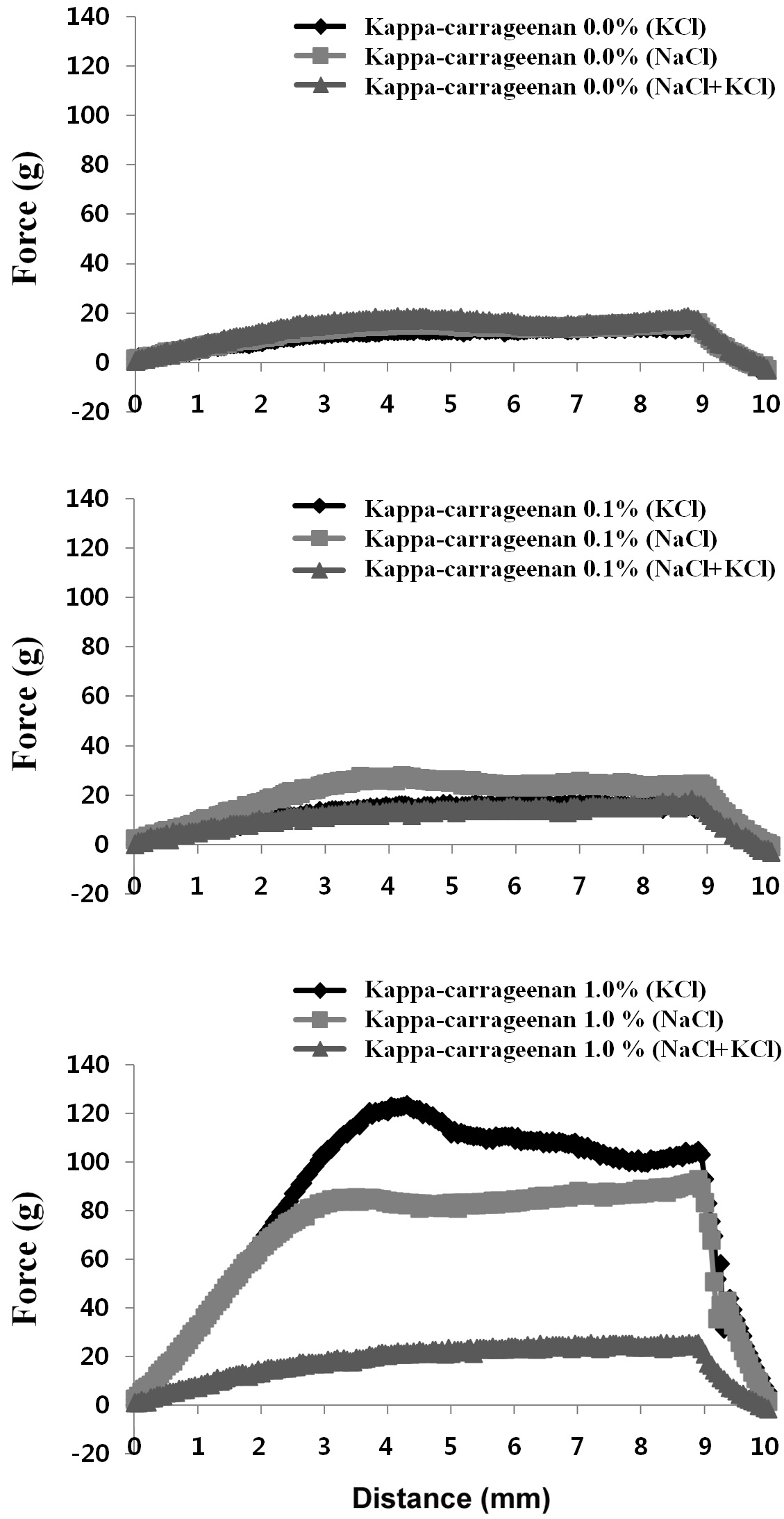
The influence of κ-carrageenan addition on the compressive properties of surimi gels containing NaCl, KCl, or the NaCl/KCl mixture was determined (Table 2). The compressive properties of surimi gels were assessed by measuring gel strength components, breaking force, deformation and gel strength; compression test results are shown in Fig. 1. The textural properties of surimi gels are controlled by the internal structure of the gel;
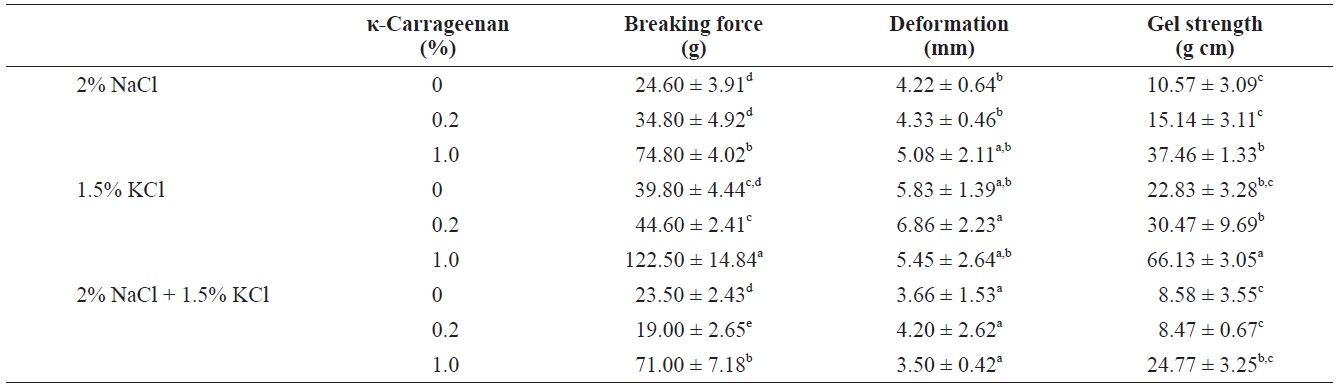
Effect of κ-carrageenan on compression property of surimi gels prepared with different salts
surimi gel. However, the NaCl/KCl mixture resulted in decreased breaking force compared to the KCl-induced surimi gel. Similar trends were observed in the strength of the surimi gels. Increasing the concentration of κ-carrageenan in surimi gels increased the gel strength, and addition of κ-carrageenan to the KCl-induced surimi gel resulted in greater strength than the NaCl-induced surimi gel. Furthermore, no particular trend of deformation was observed with increasing κ-carrageenan contents. Therefore, addition of κ-carrageenan with various salts did not improve deformation. These results indicate that as a salt source, KCl positively affects the gelling properties of the surimi gel. When comparing dynamic rheology results of salt-based fish protein systems (Hunt and Park, 2013), the addition of KCl to surimi gels with carrageenan resulted in an increased elastic modulus at a heating temperature of 80℃, compared to use of NaCl.
Carrageenan is gelatinized by a coil-helix transition. κ-carrageenan is structurally a sulfated galactan, and the addition of KCl induces the binding of alkaline ions (K+) to the κ-carrageenan helix, resulting in partial neutralization of the sulfate groups (Montero and Perez-Mateos, 2002; Ortiz and Aguilera, 2004). According to Penroj (2005), the polymer chains combine into double helices, which interlink to form relatively small domains on cooling. The domains form a three-dimensional network upon gelatinization, resulting in aggregation of the double helices and an increase in the gelling properties. In the carrageenan-induced fish protein system, competition between the fish protein and the carrageenan for the added salt and water-holding capacity occurs during heating. Additionally, the κ-carrageenan may distribute uniformly, concentrating the fish protein complex, and creating a stronger protein-hydrocolloid network in the presence of KCl, resulting in an increased gelling ability.
In conclusion, our data suggest that the gelling property of κ-carrageenan-induced Alaska pollock surimi was increased significantly by the incorporation of KCl rather than NaCl. The addition of κ-carrageenan increased the gel strength of surimi gels. Gels containing 1% κ-carrageenan and KCl had the greatest strength, while those containing 1% κ-carrageenan and NaCl showed a slightly lower strength. The addition of κ-carrageenan caused an increase in the whiteness values of the surimi gels. Further investigation is needed to better understand the role of KCl in the gelatinization of surimi gels.