Microalgae have been studied and exploited for the production of biomass or high value compounds such as glycerol, carotenoids and a variety of fine chemicals (Spolaore et al. 2006). Among such microalgae, Dunaliella are halotolerant green algae, which can grow in hypersaline aquatic environments having varied salinities from 0.5 to 5.5 M (Karni and Avron 1988, Chen and Jiang 2009, Polle et al. 2009). Some of Dunaliella strains are well known for the high production of carotenoids, especially β-carotene (Jin et al. 2003, Jin and Polle 2009). This alga produces large amount of β-carotene under stress conditions such as high light (HL) (Vorst et al. 1994, Coesel et al. 2008, Lamers et al. 2010), high salinity (Vorst et al. 1994, Hadi et al. 2008), nutrient limitation (Marin et al. 1998, Coesel et al. 2008), and nitrogen starvation (Sanchez-Estudillo et al. 2006, Lamers et al. 2012). Ability of Dunaliella to thrive under extreme salinities gives a selective advantage that inhibits growth of other algae and its predators.
β-Carotene is a red-orange pigment that belongs to isoprenoid compounds and is present in plants and algae. β-Carotene has various roles in the biological system such as precursor of provitamin A (Tanumihardjo 2002), absorption of light energy as an accessory pigment (Edwards and Walker 1983, Ben-Amotz et al. 1987), quenching of triplet state chlorophyll and singlet oxygen (Ben- Amotz et al. 1996, Ramel et al. 2012). β-Carotene is used in food (Dufosse et al. 2005), pharmaceutical industries as antioxidants (Chidambara Murthy et al. 2005), anti-tumor agent (Raja et al. 2007a, Theodosiou et al. 2010) and heart disease preventive (Tornwall et al. 2004). Because of the beneficial effects of β-carotene, the demand of β-carotene is increasing. However, about 90% of β-carotene is produced synthetically (Raja et al. 2007b).
D. bardawil has been used as commercial strain for β-carotene production. To test if the D. salina CCAP 19/18 can be used as carotenogenic strain for commercial application, the effect of light intensity on growth, morphology, photosynthetic efficiency and carotenoid content of both strains were investigated and compared. This study shows that D. salina CCAP 19/18 can be used as a carotenogenic strain for the mass production of β-carotene under a certain light regime.
D. salina CCAP 19/18 and D. bardawil LB 2538 were grown in 200 mL of artificial seawater (Pick et al. 1986) containing 1.5 M NaCl in a 500-mL Erlenmeyer flask. Cells were grown under various intensity of continuous cool white fluorescent light at 25 ± 2℃. The cultures were manually shaken occasionally. Density of the cell was measured by counting the number of cells under microscope using Neubauer hemacytometer (Superior, Bad Mergentheim, Germany). Morphology of cell was observed under Olympus microscope (BX 53; Olympus, Tokyo, Japan) and pictures were taken using CCD camera attached to the microscope (DMCe 310 plus; INS, Seoul, Korea).
Chlorophyll fluorescence was measured using FMS2 pulse-amplitude-modulation (PAM) fluorometer (Hansatech Instruments Ltd., Norfolk, UK). Dunaliella cells were dark-adapted for 10 min prior to measurement, then subjected to a 0.7 s flash of saturating white light (14,000 μmol photons m-2 s-1) to measure Fm. To measure Fm′, actinic light (150 μmol photons m-2 s-1), was applied and the saturating pulses were applied at 20 s interval.
Oxygen evolution of the culture was measured using Clark type O2 probe (Hansatech Instruments Ltd., Norfolk, UK) described in Jin et al. (2001) with slight modification. The chamber containing 2 mL of cell suspension adjusted to 2 μM chlorophyll was illuminated with a halogen lamp. The oxygen evolution was measured at 50, 85, 145, 450, 700, and 1,200 μmol photons m-2 s-1 intensity irradiated for 2 min at each light intensity. The rate of oxygen evolution at each light intensity step was recorded for 2 min.
Pigments were extracted by addition of 80% acetone with vigorous vortexing and centrifuged at 14,000 ×g for 4 min. The extract was filtered through 0.2 μm nylon filter and the filtrate was immediately subjected to Shimadzu Prominence HPLC model LC-20A (Shimadzu, Kyoto, Japan) equipped with a Waters Spherisorb S5 ODS1 4.6 × 250 mm cartridge column (Waters, Milford, MA, USA). The pigments were separated using a solvent mixture of 0.1 M Tris-HCl pH 8.0, acetonitrile, methanol, and ethylacetate. During the run, the solvent concentrations were 14% 0.1 M Tris-HCl, 84% acetonitrile, and 2% methanol from 0 to 15 min. From 15 to 19 min, the solvent mixture was consisted of 68% methanol and 32% acetonitrile. A post-run was performed for 6 min with the initial solvent mixture. The flow rate was constant at 1.2 mL per min. Pigments were detected at 445 nm. Concentration of the individual pigment was determined from the HPLC profiles calibrated with standard pigments of chlorophyll and carotenoids (14C Centralen; DHI, Hørsholm, Denmark).
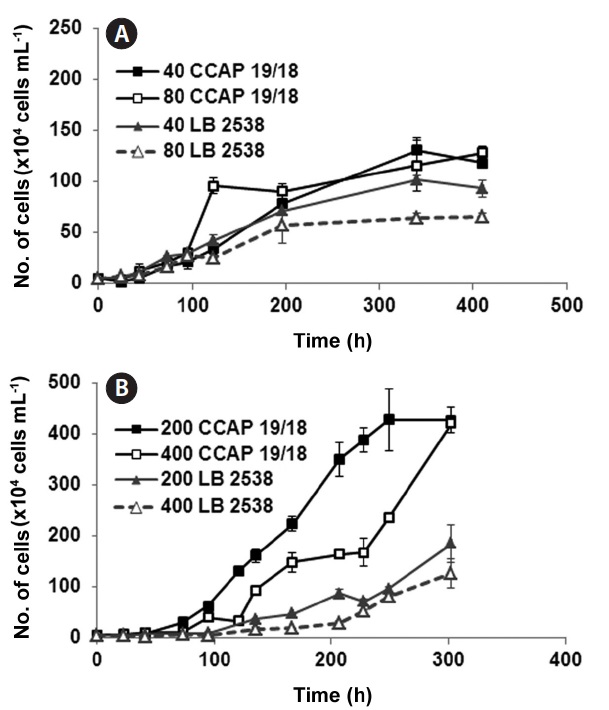
To investigate the effect of light intensity on the growth of D. salina CCAP 19/18 and D. bardawil, low light (LL) (40 and 80 μmol photons m-2 s-1) and high light (HL) (200 and 400 μmol photons m-2 s-1) were tested. The intensity of light significantly affected both cell density and doubling time of the culture (Fig. 1). Under LL growth conditions, growth of D. bardawil under 80 μmol photons m-2 s-1 of light was lower than that under 40 μmol photons m-2 s-1 of light (Fig. 1A). There was no difference in growth of D. salina CCAP 19/18 under both LL light conditions, except on day 5 (120 h) when the growth under 80 μmol photons m-2 s-1 of light was higher than that under 40 μmol photons m-2 s-1 of light (Fig. 1A). Both strains showed saturation
of growth after 14.6 days (350 h) (Fig. 1A). Under HL conditions, the growth rate of D. bardawil under 400 μmol photons m-2 s-1 of light was slightly lower than that of under 200 photons m-2 s-1 (Fig. 1B). The growth of D. salina CCAP 19/18 was slower under 400 μmol photons m-2 s-1 than that under 200 μmol photons m-2 s-1 of light. D. salina CCAP 19/18 showed faster and higher growth during the 300 h period than D. bardawil under HL growth conditions (Fig. 1B).
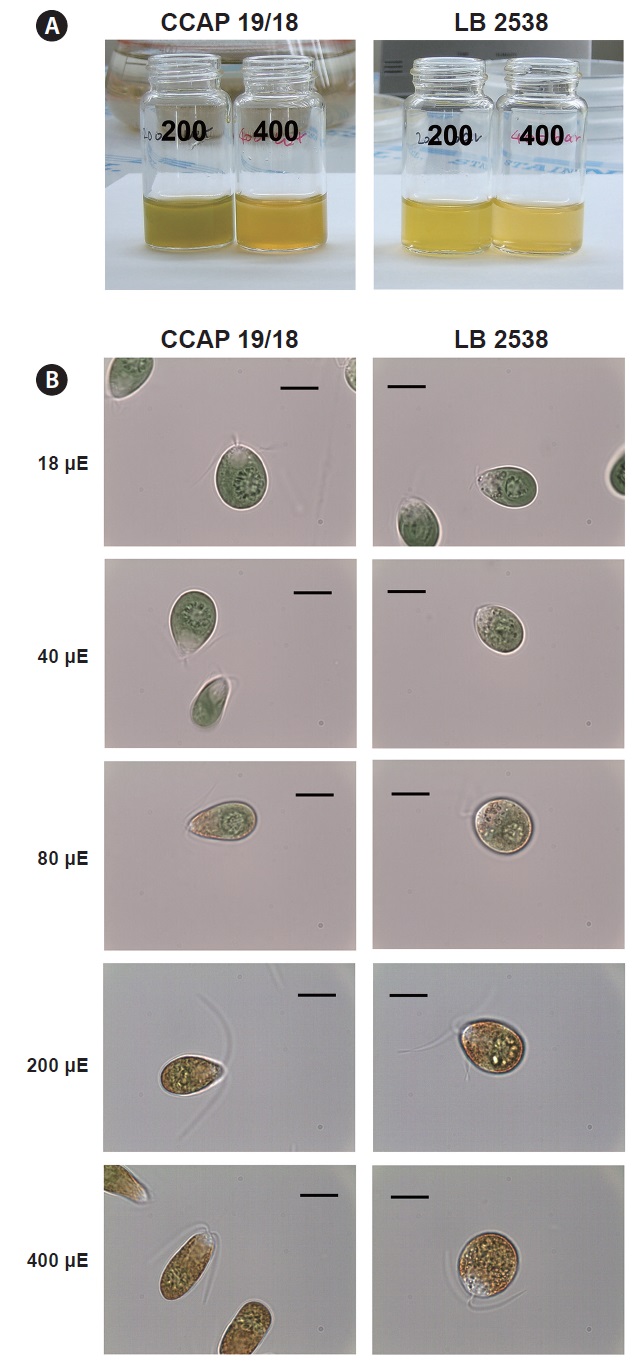
We compared the color and morphological change under HL conditions between D. salina CCAP 19/18 and D. bardawil. Under 200 μmol photons m-2 s-1, D. bardawil had more yellowish color than D. salina CCAP 19/18 (Fig. 2A). D. salina CCAP 19/18 had yellow green color at 200 μmol photons m-2 s-1 (Fig. 2A). Under 400 μmol photons m-2 s-1, both D. bardawil and D. salina CCAP 19/18 had yellow color, but D. salina CCAP 19/18 had more orange color (Fig. 2A).
There were changes in morphology under various light
intensities. Under 18 and 40 μmol photons m-2 s-1 of light, both D. bardawil and D. salina CCAP 19/18 had ovoid shape (Fig. 2B). Under 80 μmol photons m-2 s-1 of light, D. salina CCAP 19/18 cells were elongated and D. bardawil cells became larger. From 80 to 400 μmol photons m-2 s-1,
both strains started to show yellowish orange pigments in the cell and the color was intensified (Fig. 2B). Under 400 μmol photons m-2 s-1 of light, D. bardawil cell was almost round and D. salina CCAP 19/18 had an elongated shape than that of D. bardawil (Fig. 2B).
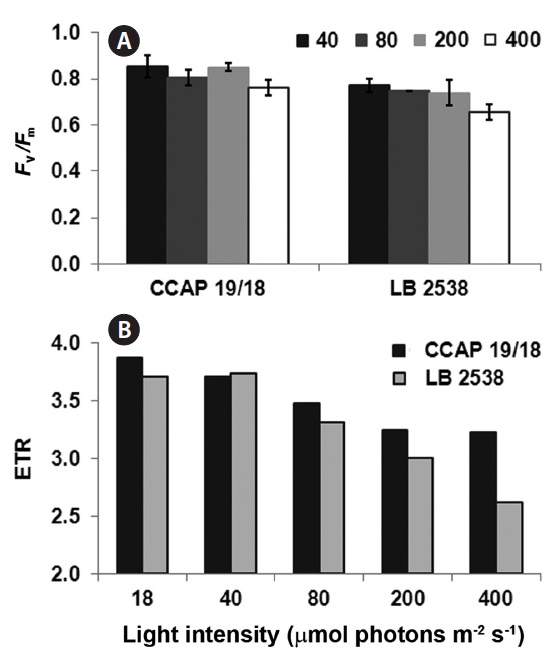
To investigate the difference in photochemical conversion efficiency of photosystem II (PSII) between D. bardawil and D. salina CCAP 19/18 under various light conditions, the maximum quantum yield of PSII (Fv/Fm) and the electron transport rate (ETR) were measured. Decrease of the Fv/Fm value indicates the extent of photoinhibition in Dunaliella (Hofstraat et al. 1994, Gordillo et al. 2001). In both strains, there was little change in Fv/Fm from 40 to 200 μmol photons m-2 s-1 light treatment (Fig. 3A). However, under 400 μmol photons m-2 s-1 of light, there was a small but significant decrease in Fv/Fm in both strains (Fig. 3A). Fv/Fm of D. salina CCAP 19/18 was slightly higher than that of D. bardawil under 400 μmol photons m-2 s-1 of light (Fig. 3A). ETR is one of an indicator of stress effects and correlates with gross photosynthetic rate. ETR was decreased in both strains when the light intensity increased (Fig. 3B). Interestingly, the ETR of D. salina CCAP 19/18 was higher than that of D. bardawil above 80 μmol photons m-2 s-1 of light (Fig. 3B). Under 400 μmol photons m-2 s-1 of light, ETR of D. bardawil was 50% of that of D. salina CCAP 19/18 (Fig. 3B). Therefore, decline in ETR of D. bardawil reflects the reduction in photosynthetic ability under the HL condition compared to D. salina CCAP 19/18.
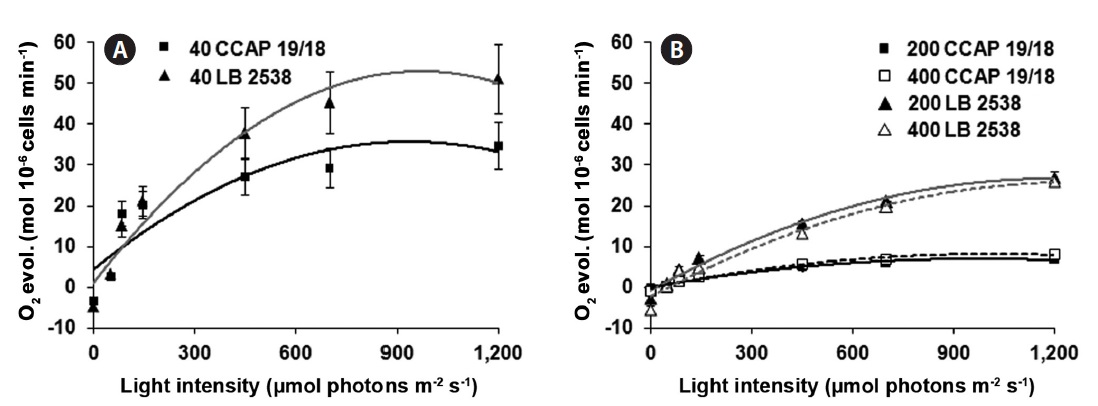
The efficiency of photosynthesis (photosynthetic capacity) was assessed to analyze the light saturation curve of photosynthesis in the two strains. For this purpose, the rate of O2 evolution was measured and plotted as a function of incident light intensity. The photosynthetic capacity on the cell basis was also saturated at 900 μmol photons m-2 s-1 of light (Fig. 4A). Under the same LL growth condition, D. bardawil showed about 1.5 times higher rate of O2 evolution on the cell basis than the D. salina CCAP 19/18 at 1,200 μmol photons m-2 s-1 of light (Fig. 4A). Under the HL growth conditions, the rate of O2 evolution on the cell basis was lower than that of under the LL growth condition (Fig. 4A & B). In case of D. bardawil, the rate of O2 evolution on the cell basis under the HL growth conditions (200 and 400 μmol photons m-2 s-1) was 2 times lower than that of under the LL condition at 1,200 μmol photons m-2 s-1 of light irradiance (Fig. 4A & B). However, in case of D. salina CCAP 19/18, the rate of O2 evolution on the cell basis under the HL growth conditions at 1,200 μmol photons m-2 s-1 of light irradiance was 4.4-5.2 times lower than that under the LL growth condition at 1,200 μmol photons m-2 s-1 of light irradiance (Fig. 4A & B).
One of the parameters of irradiance stress response in green algae is the de-epoxidation state of xanthophylls (Niyogi 1999, Jin et al. 2001). The xanthophyll cycle plays a significant role in the non-photochemical quenching (NPQ) of excitation and the photoprotection of the photosynthetic apparatus (Yamamoto 1979, Muller et al. 2001). In general, under the LL condition, cells accumulate violaxanthin and have low level of zeaxanthin. Under the HL stress condition, cells de-epoxidize violaxanthin to zeaxanthin by violaxanthin de-epoxidase (Muller et al. 2001). Zeaxanthin is known to quench the excited Chl*
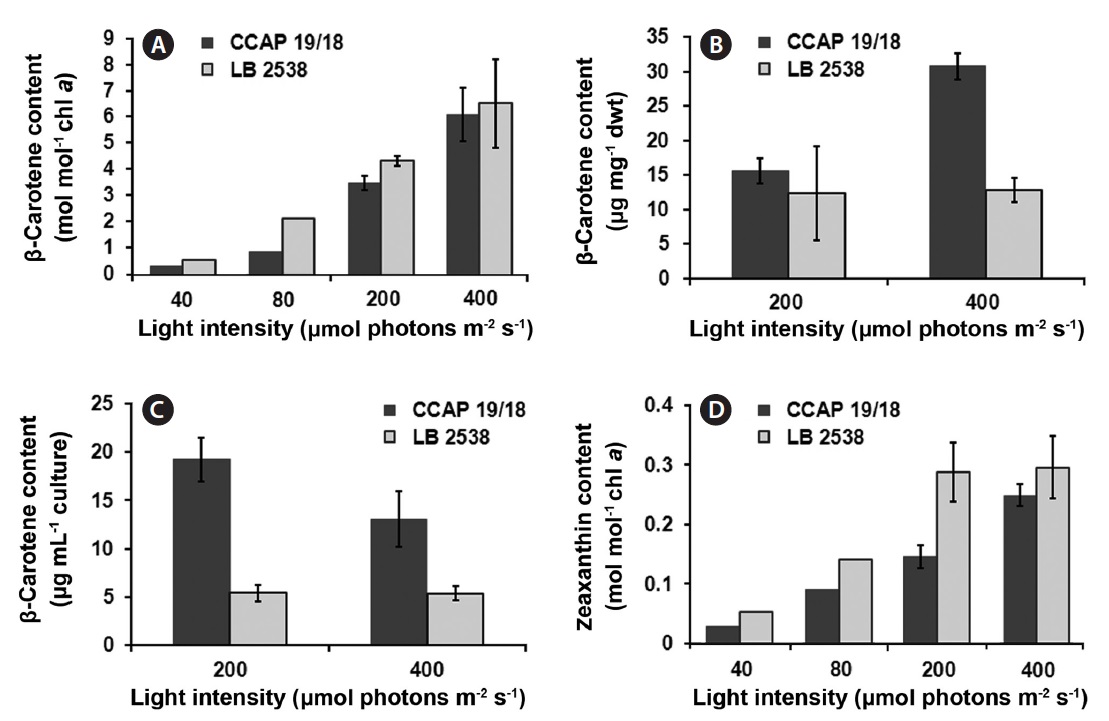
molecules (Baroli and Niyogi 2000, Frank et al. 2000, Muller et al. 2001). Therefore, analyzing the components in the xanthophyll cycle is to understand the photoacclimation and photoinhibition process in algae (Baroli and Melis 1996). We addressed a question whether the difference in the de-epoxidation state exists between the two strains under various light conditions. The content of β-carotene and zeaxanthin was estimated on a per chlorophyll a basis. The β-carotene content of both strains increased when the light intensity increased (Fig. 5A). The β-carotene content of D. bardawil was higher than that of D. salina CCAP 19/18 under all light intensities and the largest difference between the two strains occurred at 80 μmol photons m-2 s-1 (2.37 times difference) (Fig. 5A). Under 400 μmol photons m-2 s-1 of light, the β-carotene content of D. salina CCAP 19/18 was about 14% less than that of D. bardawil. Because the actual yield of β-carotene in D. salina CCAP 19/18 was much higher than that of D. bardawil due to much higher growth rate of D. salina CCAP 19/18, the β-carotene yield on the dried mass basis of the cells and the culture volume basis grown under HL was measured (Fig. 5B & C). There was little difference in the β-carotene content on the dry weight basis between the two species under 200 μmol photons m-2 s-1 of light. However, the β-carotene content on the dry weight basis of D. salina CCAP 19/18 was 2.4 times higher than that of D. bardawil under 400 μmol photons m-2 s-1 of light (Fig. 5B). On the culture volume basis, the β-carotene content of D. salina CCAP 19/18 was 3.8 and 2.5 times higher than that of D. bardawil under 200 and 400 μmol photons m-2 s-1 of light, respectively (Fig. 5C). Zeaxanthin content also showed increase in amount in response to the increasing light intensity in both species, and the amount of zeaxanthin content of D. bardawil was about 2 times higher than that of D. salina CCAP 19/18 except at 400 μmol photons m-2 s-1 of light (Fig. 5D). The largest difference in amount of zeaxanthin content occurred at 200 μmol photons m-2 s-1 of light (1.97 times difference between two strains).
In this report, we compared the growth kinetics, morphological change, parameters of photosynthetic rate (rate of O2 evolution, Fv/Fm and ETR) and the production of carotenoids in response to various intensity of light between two Dunaliella strains, D. salina 19/18 and D. bardawil.
In both strains, the growth was decreased as the light intensity increased (Fig. 1). The two strains showed different growth kinetics under HL growth conditions, but similar growth kinetics under LL growth condition (Fig. 1A & B). There were changes in morphology in response to light intensity and both strains showed difference. On one hand, D. salina CCAP 19/18 became more elongated as the light intensity increased and it was accompanied with the increase in cell number under HL growth condition (Figs 1B & 2B). On the other hand, D. bardawil cell seemed to be increased in volume as the light intensity increased, and the cell division was less than that of D. salina CCAP 19/18 (Figs 1B & 2B). This difference in the cell division rate and volume change might result in the difference in the O2 evolution rate on the cell basis between both strains. Little difference in the O2 evolution rate on per chlorophyll basis between the two strains implies that the degradation rate of chlorophyll of both strains might be similar in the range of light intensity investigated. The equiproportional increase in the production rate of cell volume and β-carotene was observed (Lamers et al. 2010). Also, β-carotene level can be regulated by the total amount of irradiation perceived during the cell division cycle (Ben-Amotz and Avron 1983, Lers et al. 1990).
According to the result of Fv/Fm (Fig. 3A) and ETR (Fig. 3B), D. salina CCAP 19/18 might be less liable to photoinhibition than D. bardawil under HL growth conditions. D. bardawil had about 1.5 and 5 times higher oxygen evolution rate than D. salina CCAP 19/18 under LL condition (Fig. 4A) and HL conditions (Fig. 4B), respectively. This implies that D. bardawil is more effective in photosynthesis than D. salina CCAP 19/18. Both strains accumulate large amount of β-carotene under HL growth condition and there is little difference between the two strains on per chlorophyll basis (data not shown). The increase of β-carotene content of D. salina CCAP 19/18 by HL matches the result in Lamers et al. (2010). The zeaxanthin production of D. bardawil was saturated at 200 μmol photons m-2 s-1 of light and that of D. salina CCAP 19/18 was not (Fig. 5B). The amount of zeaxanthin in the cell is known to have close relationship with the NPQ, and NPQ is one of parameters of photoprotection (Demmig-Adams and Adams 1992, Muller et al. 2001).
Previously, similar works were performed and reported (Ben-Amotz and Avron 1983, Vorst et al. 1994, Gomez and Gonzalez 2005). However, in Ben-Amotz and Avron (1983) and Vorst et al. (1994), the D. salina strain they used was not able to accumulate β-carotene. In Gomez and Gonzalez (2005), they used the same strain as we did. However, the HL they used was 110 μmol photons m-2 s-1, which was an intermediate intensity compared to that we used. The β-carotene production of D. salina CCAP 19/18 and D. bardawil was not different under 40 and 110 μmol photons m-2 s-1 of light, which was different from our result (Gomez and Gonzalez 2005). This might be due to the difference in culture condition. Recently, the effect of light intensity on β-carotene production was performed with D. salina CCAP 19/18 and they also showed the increase in β-carotene content by HL (Lamers et al. 2010).
Development in biotechnology and bioengineering encouraged invention of many systems required to facilitate cultivation of Dunaliella and harvesting β-carotene in an industrial scale. Efforts for effective production of β-carotene include isolation of carotenogenic strain (Markovits et al. 1993), cloning of genes involved in carotenogenesis (Zhu et al. 2008), transformation of these genes into microalgae (Leon-Banares et al. 2004, Walker et al. 2005), searching for optimum environmental stimuli in β-carotene accumulation (Ben-Amotz and Avron 1983, Sanchez-Estudillo et al. 2006). These efforts, however, did not surpass the systematic technical development such as two-phase bioreactors (Hejazi et al. 2004). Because environmental stress such as high irradiance inhibits Dunaliella growth, even causes photo-bleaching, two separate steps of culture is required i.e., one for growth and one for β-carotene accumulation (Hejazi et al. 2004). Also, there is an inverse relationship between β-carotene content and the growth rate (Ben-Amotz et al. 1982). In this study, D. salina CCAP 19/18 has higher growth rate in D. bardawil at 200 μmol photons m-2 s-1 of light (Fig. 1B) and the same strain showed higher β-carotene content even under 2,000 μmol photons m-2 s-1 of light than LL growth condition (Lamers et al. 2010). Setting the light condition for the maximal growth rate and for the overproduction of β-carotene is indispensable for scaling up to the industrial scale both in the batch culture and turbidostat culture. In this sense, D. salina CCAP 19/18 is more beneficial to the carotenogenic D. bardawil because of its higher growth rate under HL condition and less liability to the light stress than D. bardawil. The growth of D. salina CCAP 19/18 was 3 times higher than that of D. bardawil and also 16% higher in Fv/Fm (Figs 1B & 3A). Furthermore, D. salina CCAP 19/18 is more ‘milkable’ than any other Dunaliella strain including D. bardawil (Kleinegris et al. 2010). Lamers et al. (2010) also showed that β-carotene productivity of D. salina CCAP 19/18 was much higher than the average productivity by commercial production facility and that the β-carotene production was closely linked to the accumulation of C16:0 and C18:1 fatty acids. We propose that there is a possibility of improvement of the β-carotene production if the beneficial characteristics of D. salina CCAP 19/18 over D. bardawil are taken advantage of under certain light condition. D. bardawil has advantage in the production of β-carotene in an open pond, whereas D. salina CCAP 19/18 might be better than D. bardawil in the bioreactor at optimized condition.
It is well known that HL induces the overproduction of β-carotene on the transcriptional and translational level (Lers et al. 1990). However, the genes and enzymes for carotenogenesis and its regulatory mechanisms are not well understood (Jin and Polle 2009, Ramos et al. 2011). Genetic modification followed by screening of the Dunaliella strains have been explored for finding novel genes for the mass production of β-carotene (Jin and Melis 2003, Ramos et al. 2011). Applying high through-put molecular genetics technique to this precious microalga D. salina CCAP 19/18 will improve the output of carotenogenesis.