Identification of individual fish is essential for growth, migration, mortality, stock identification, and gear selectivity to trace particular fish populations. Although short-term tag retention may suffice for some experiments, the effect of a tag on fish survival, behavior, growth, recognition, and cost of the marking technique should be considered. However, traditional external tags (such as spaghetti or dart tags) are often lost quickly (Crossland, 1980; Bergman et al., 1992) and may affect growth or survival (Crossland, 1976; Tong, 1978; Mc- Farlane and Beamish, 1990; Serafy et al., 1995). Furthermore, these types of tags can only be read by recapturing the fish.
Internally situated but externally readable devices such as acoustic tags are often limited by their expense. Problems associated with the physiological characteristics of the fish, reliability of identification, fouling of the tag by algae (Jones, 1987; Barrett, 1995), tag retention (Crossland, 1976; Parker, 1990), and external visibility have reduced the confidence of in situ studies of reef fish ecology.
Many fish species have transparent tissue suitable for tagging, including opecula, mandible, top of the head, body, and fins. However, sites to retain tags vary among species. Tagging sites in other body locations may also be used successfully. An example of a visual mark that has been successfully applied to several fish species is the visible implant fluorescent elastomer
(VIE) tag (Northwest Marine Technology, Ltd., Shaw Island, WA, USA). It was developed primarily for tagging large batches of small or juvenile fishes. The VIE is comprised of a viscous liquid elastomer that sets into a pliable solid over a period of hours. The elastomer can be injected into transparent or translucent tissues to form a permanent, biocompatible mark. The compound fluoresces brightly when exposed to blue light (UV lamp) and viewed through an amber filter.
Tag size can easily be varied according to the requirements of the researcher and the size of the fish to be tagged. This system has been used to identify groups or cohorts of juvenile reef fishes (Frederick, 1997) and salmonids (F. Haw personal communication) but is also proving potentially effective for controlled laboratory studies of adults (Dewey and Zigler, 1996). As an externally visible but subdermally situated marking system, VIE tags are potentially able to eliminate many of the problems experienced with other methods and can be used to trace individual fish.
Greenling
The fish used in this experiment were sub-adult greenling
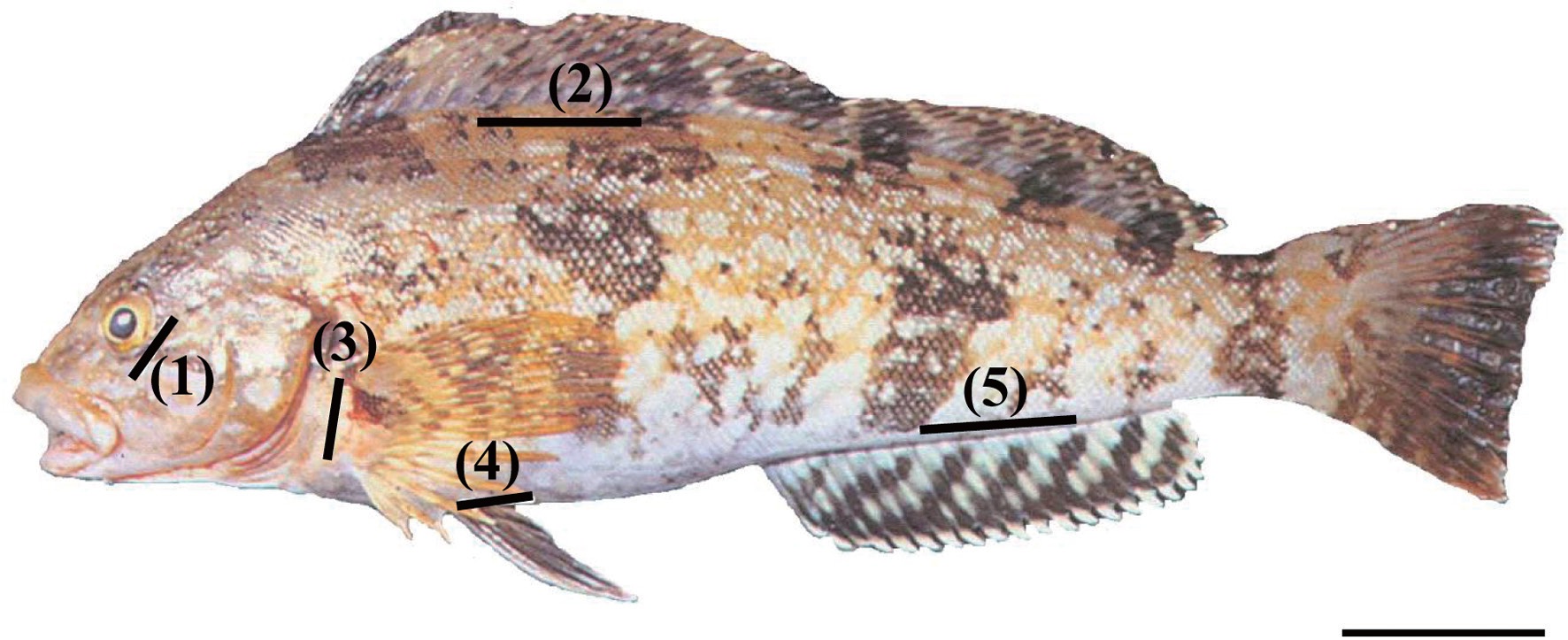
Fifty fish in triplicate groups were individually marked with orange, yellow, red, or green elastomer at five body locations (Fig. 1): 1) the adipose eyelid, 2) the surface of the dorsal fin base, 3) the inside surface of the pectoral fin base, 4) the inside surface of the pelvic fin base, and 5) the surface of the anal fin base. Control fish (
The fish were held in 18 flow-through fiberglass-reinforced plastic tanks (2 × 2 × 0.5 m; water volume, 2,000 L) supplied with filtered seawater. The bottom of the tanks was coated with a black sheet to facilitate decoding of the tags. Flow rate was 100 L min-1 tank-1, and mean water temperature was 20 ± 2.5℃ respectively. A day-night cycle was established, and all tanks were covered with mesh to prevent fish jumping out. The fish were fed daily to satiation with dry commercial flounder feed (Agribrand Furina Korea Co., Seoul, Korea) throughout the 20-month trial.
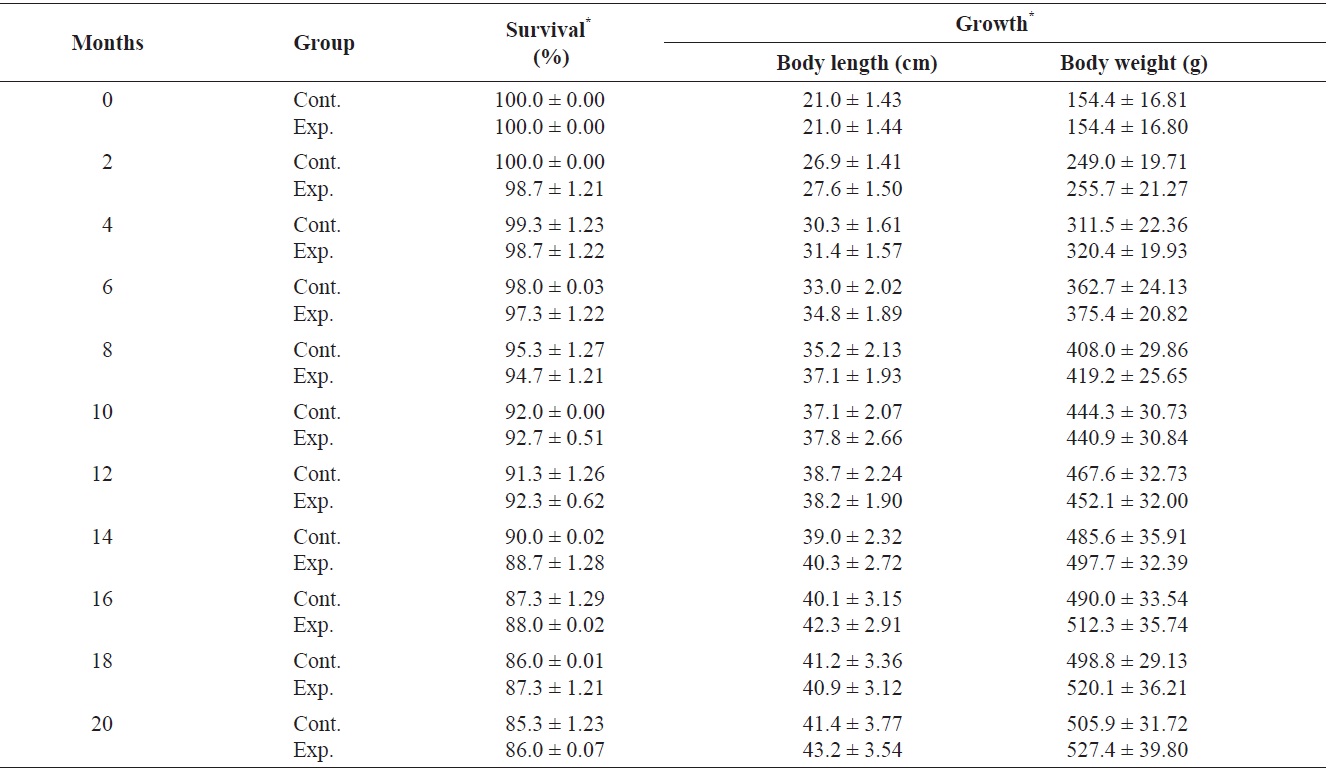
Growth of fish and retention, underwater visibility, and readability of the tags were determined at 2-month intervals, and dead fish were removed daily. Only individuals marked with color on the dorsal fin could be distinguished more than 2 m away by eye and with a blue light (UV lamp) after 2 months. Tag retention rates were calculated according to the method of Zerrenner et al. (1997). The mark-retention data recorded from dead fish were used to calculate the percent retention up to the date on which they died, but were not used in subsequent calculations.
Data are expressed as means of triplicate experiments. Differences in survival and growth between the control and experimental groups were assessed by the
Survival and growth of greenling
The primary causes of mortality in caged snapper tagged with the same system are internal damage and infection from gas bladder rupture, or infection from anatomical trauma caused by handling (Willis and Babcock, 1998). Greenling was less fragile; however, steps should be taken to minimize the possibility of capture-related injury. Therefore, skilled application of the elastomer injection was crucial for maintaining low mortality, as suggested by the decrease in mortality of marked fish during a laboratory experiment (Frederick, 1997). Jang et al. (2007) investigated the effectiveness of passive integrated transponder (PIT) tags in a small cyprinid
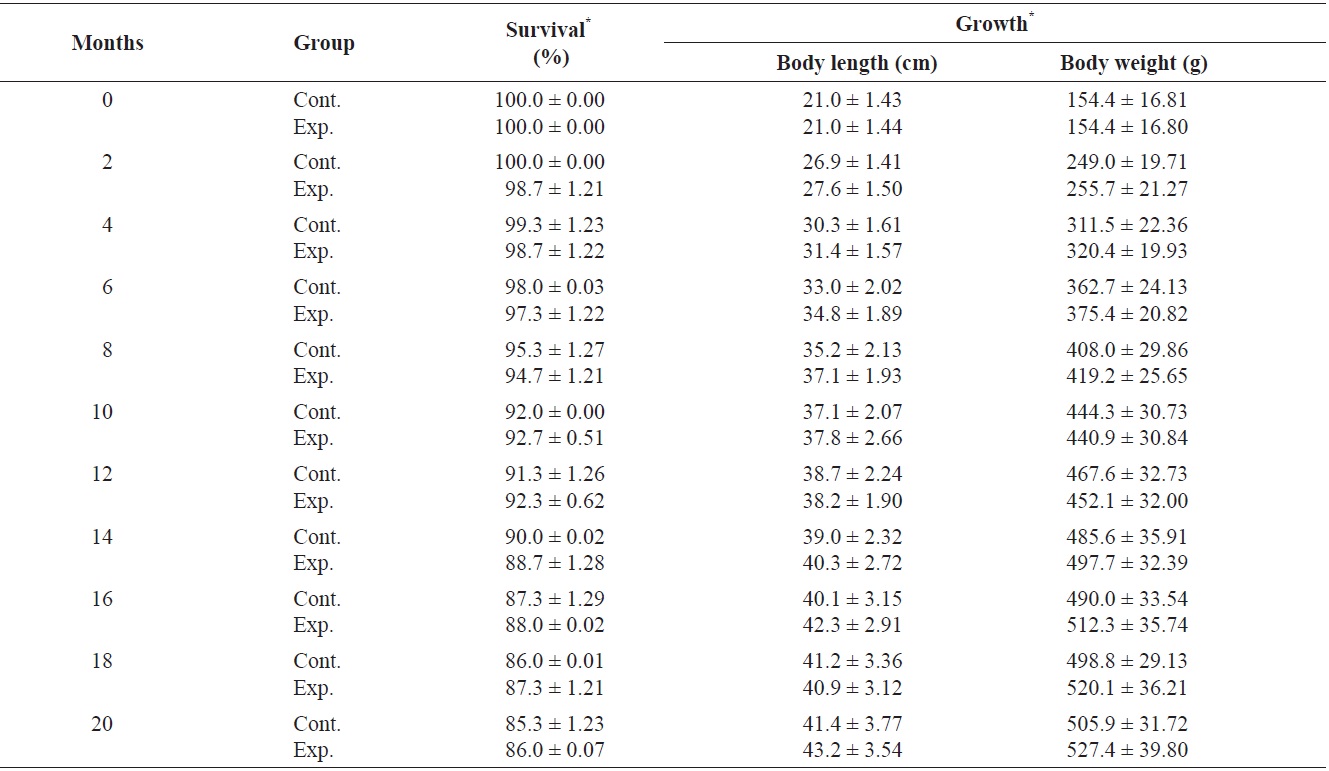
Survival (%) and growth in greenling Hexagrammos otakii from 0 to 20 months after visible implant fluorenscent elastomer (VIE) tagging
1.1 cm; mean body weight ± SD, 71.7 ± 0.2 g). Upon use of three types of tags, including small PIT tags (width, 11.0 mm; height, 2.1 mm; weight, 0.088 g), mid-sized PIT tags (width, 20.0 mm; height, 3.5 mm; weight, 0.188 g), and large PIT tags (width, 30.0 mm; height, 3.5 mm; weight, 0.298 g), survival rates of 100 individuals 30 days after tag insertion were as follows: large tag, 50.0%; middle tag, 57.5%; and small tag, 61.4%. Survival rates varied among the three tag types because death was due to surgical damage. However, we used the VIE tag which resulted in a high survival rate and no surgical damage to the fish.
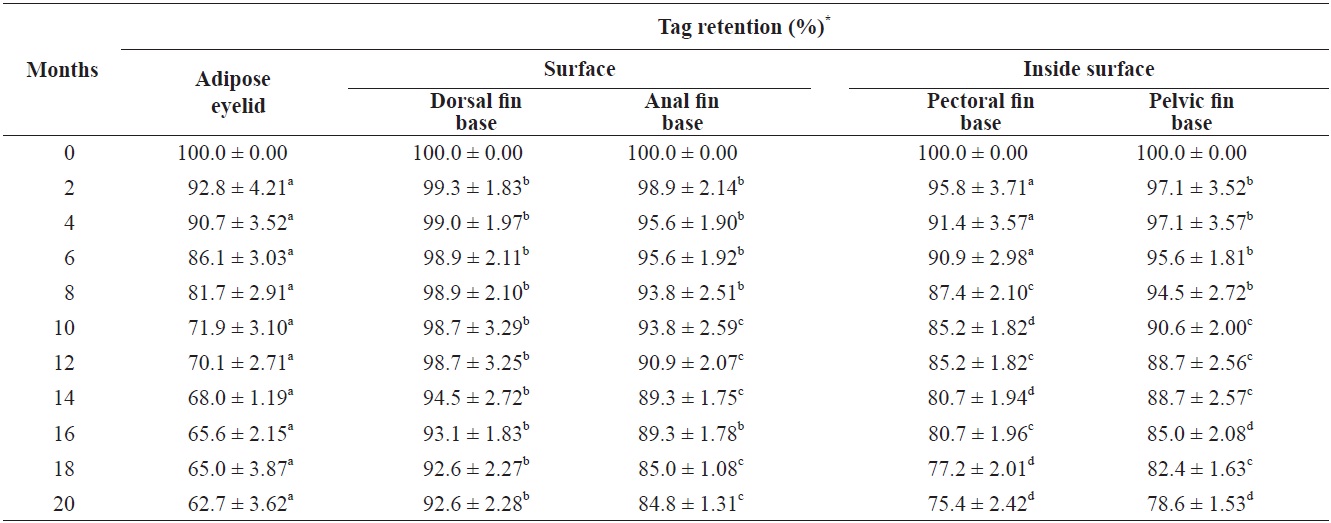
Greenling retained > 90% of the tags at the surface of the dorsal fin base (Table 2), which is similar to previous VIE studies (Dewey and Zigler, 1996; Willis and Babcock, 1998). The anal fin base showed a higher tag retention rate than marking the inside surface of the pectoral fin and pelvic fin bases (
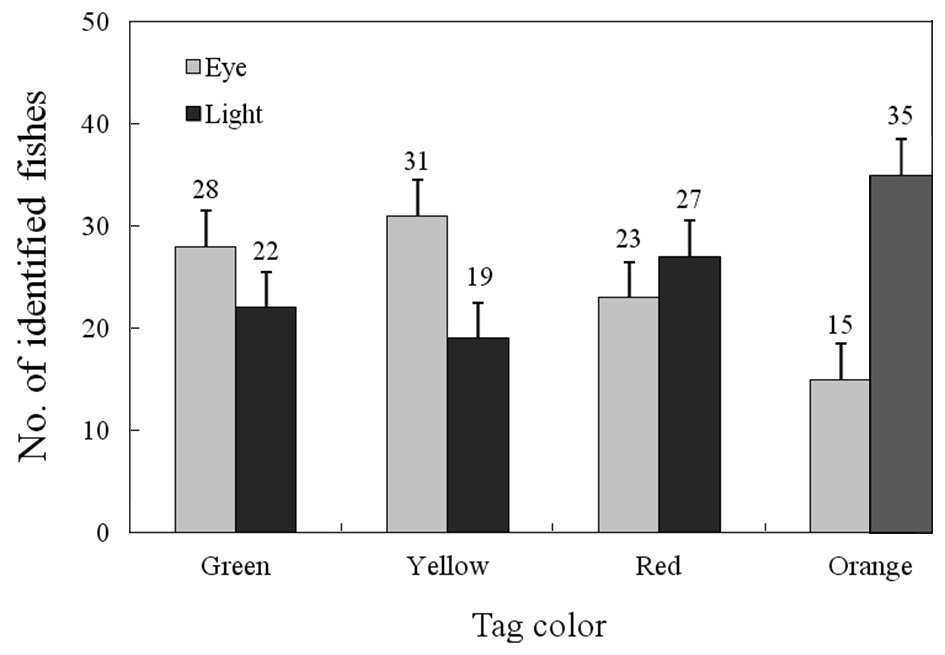
Tags of different colors were not equally identifiable underwater (Fig. 2). Only individuals marked with color on the
dorsal fin were distinguishable at distances > 2 m by eye or with a UV lamp. Twenty-eight fish tagged with green were identifiable with the naked eye, and 22 were with a UV lamp. Thirty-one fish tagged with yellow were identifiable with the naked eye, and 19 with a UV lamp. Twenty-three fish tagged with red were identifiable with the naked eye, and 27 with a UV lamp. Fifteen fish tagged with orange were identifiable with the naked eye, and 35 with a UV lamp. These results are similar to those of a previous study (Willis and Babcock, 1998).
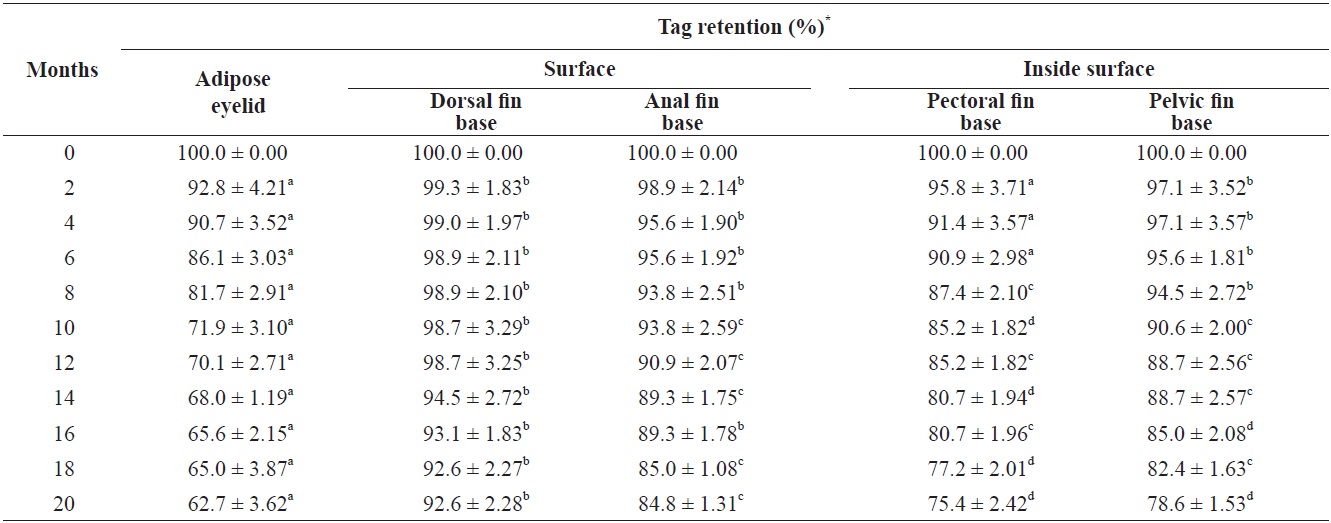
Retention rate (%) of visible implant fluorenscent elastomer (VIE) tags in each sites of greenling Hexagrammos otakii from 0 to 20 months after VIE tagging
Significant differences were found among tag colors; red and orange were easier to detect than green and yellow using a UV lamp. Green and yellow could be detected easily with the naked eye. The method of detection (naked eye
Buckley et al. (1994) found that red monofilament micro tags were difficult to detect underwater because of the attenuation of short wavelength light. However, we could more easily detect red compared to green or yellow (Willis and Babcock, 1998). All dives in this study were limited to 2 m. Deeper water where natural light levels are lower may result in greater attenuation of red light (Willis and Babcock, 1998). Red tags are clearly recognizable at up to 5 m in clear water under direct sunlight (Willis and Babcock, 1998).
As mentioned by Willis and Babcock (1998), a variety of factors may influence the distance at which tags can be positively identified. It was often possible to recognize a fish as possessing a tag at > 6 m without being able to detect tag color. The direction and intensity of ambient light made a large difference; when the sun was low, and behind the observer, tag color could be correctly identified at a greater distance. The amount of suspended material was also a contributing factor, particularly in the effectiveness of fluorescence. Backscatter from large particles as well as strong ambient light decreases the effective range of a UV lamp beam, thereby decreasing the range of detection. The range of detection of orange tagged fish using a UV lamp and amber filter appears to be limited primarily by the water clarity and the power of the light used (Willis and Babcock, 1998). We found that the VIE mark was easy to apply, requiring < 1 min per fish, and was readily visible when viewed under an UV lamp in greenling, a benthic species.