Quinone reductase (QR), one of the representative phase II enzymes, is a flavoprotein that catalyzes the reduction and detoxification of electrophilic quinones and quinone derivatives. QR has been shown to protect mammalian cells from redox cycling, oxidative stress, and neoplasia by converting toxic quinines to hydroquinones and inducing other detoxifying enzyme such as glutathione-, glucuronidyl-, and sulfotransferase enzymes in response to xenobiotics, antioxidants, oxidants, heavy metals, and radiation (Talalay et al., 1995; Song et al., 1999; Jaiswal, 2000). Therefore, upregulation of QR is thought to be a useful biomarker of phase II metabolic activity and carcinogenic elimination (Moon et al., 2006). Measuring the QR induction activity in cultured Hepa1c1c7 cells is a simple screening method for detecting the capacity of compounds to act as potential chemopreventors (Prochaska, 1994). Hepa1c1c7 cells are a suitable model for studying chemoprotection because they maintain many characteristics of normal tissue including the capacity for carcinogen activation and metabolism in response to environmental, nutritional, and hormonal factors (De Long et al., 1986).
Many polyphenols including flavonoids from terrestrial plants have been shown to modulate the phase II enzyme detoxification pathway, a mechanism implicated in their chemopreventive action (Lampe, 2003; Chen and Blumberg, 2008; Yang and Liu, 2009). However, the phase II enzyme activity of phlorotannins from
In the present study, we investigated the QR induction activity of phlorotannins derived from
Dried leafy thalli of
>
Extraction and isolation of phlorotannins
The dried powder of
>
Cell culture and cytotoxicity determination
Hepa1c1c7 cells (mouse hepatoma cells; American Type Culture Collection, Manassas, VA, USA) were cultured and maintained in α-MEM (Gibco BRL/Life Technologies) containing 100 μg/mL penicillin streptomycin and 10% FBS, and maintained at 37℃ under a humidified atmosphere with 5% CO2.
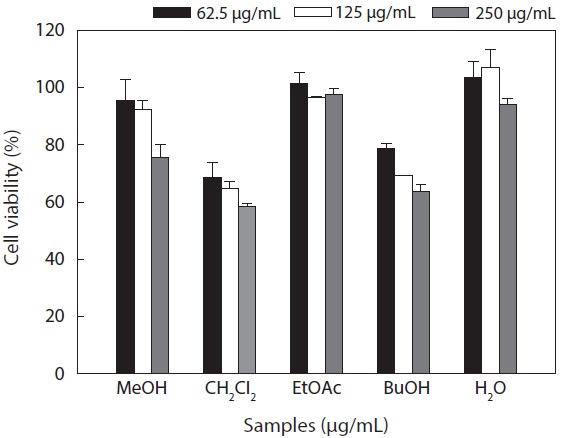
Cytotoxicity levels of the MeOH extract and its solvent soluble fractions of
The QR induction bioassay was modified from a method described previously (Prochaska and Santamaria, 1988; Zhang et al., 1992). Hepa1c1c7 murine hepatoma cells were grown in α-MEM without nucleosides or deoxyribonucleosides and supplemented with 10% FBS at 37℃ in an atmosphere of 5% CO2 in a humidified incubator. Cultured Hepa1c1c7 cells were plated in 12-well plates and decanted after 24 h incubation. Fresh medium and test samples dissolved in 10% DMSO were serially introduced. The final DMSO concentration in the medium was less than 0.5%. The cells were incubated for an additional 48 h. Sulforaphane was used as a positive control. The medium was removed, the cells were lysed with 50 μL of 0.8% (w/v) digitonin in 2 mM EDTA at pH 7.6 and incubated for 10 min at 37℃. The plates were then agitated on a shaker (120 rpm) for 10 min at room temperature. A 200 μL aliquot of reaction solution (7.5 mL of 0.5 M Tris HCl buffer, pH 7.4; 100 mg of albumin from human serum; 1 mL of 1.5% Tween 20 solution; 0.1 mL of 7.5 mM FAD; 1 mL of 150 mM glucose 6-phosphate; 90 μL of 50 mM NADP; 300 U of yeast glucose 6-phosphate dehydrogenase; 45 mg of MTT; 150 μL of 50 mM menadione [as an oxidant] in acetonitrile; and 140.16 mL distilled water added to a total volume of 150 mL) was added to lysed cells. Menadione solution was added just before the mixture was dispensed into the microtiter plates. Readings were made in triplicate for each sample at 590 nm. Total protein concentrations were determined in a duplicate set of plates using crystal violet staining and subsequently scanned at 490 nm (Prochaska et al., 1992). The specific activity of QR is defined as nmol MTT blue formazan reduced per min and per mg protein (De Long et al., 1986). Induction was expressed as the ratio of the specific activity of QR in the presence and absence of the test sample.
Here, 3,345 nmol/mg is the ratio of the proportionality constant determined for crystal violet and the extinction coefficient of MTT.
Cancer chemoprevention has been recognized as the administration of chemicals or dietary components to interdict, suppress, or reverse the development of cancer in normal or preneoplastic tissue (Sporn and Newton, 1979; Wattenberg, 1985). As a new strategy for cancer chemoprevention, the induction of phase II drug-metabolizing enzymes like QR is a principal mechanism of protection against chemical stress and the initiation of carcinogenesis (Talalay et al., 1995). QR induction blocks the generation of mutagenic quinone metabolites and diminishes the covalent conjugation of oxygenated metabolites to microsomal proteins (Prochaska et al., 1985), and this enzyme maintains the capacity of the cells to survive the stress of oxidative metabolites (Talalay and Benson, 1982; Ysern and Prochaska, 1989; Forrest et al., 1990; Merk et al., 1991; Dicker and Cederbaum, 1993).
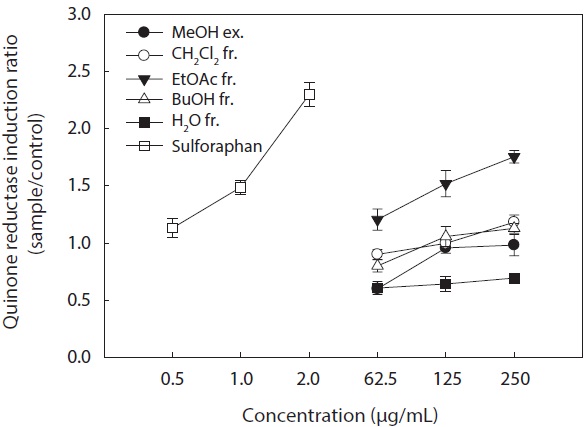
In the present study, we investigated the QR induction activities of the MeOH extract and its organic solvent fractions derived from
Hepa1c1c7 cells at a concentration of 250 μg/mL. However, the CH2Cl2 and
These results suggested that the active ingredients of
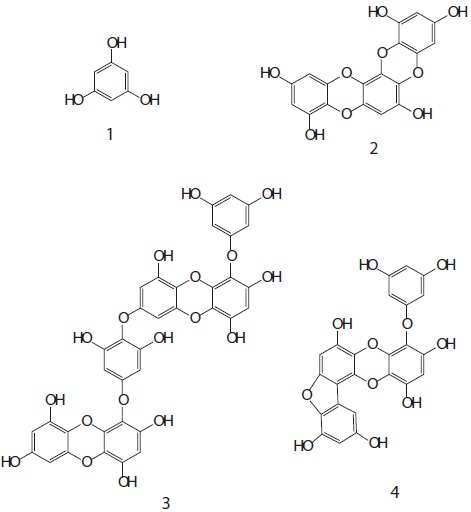
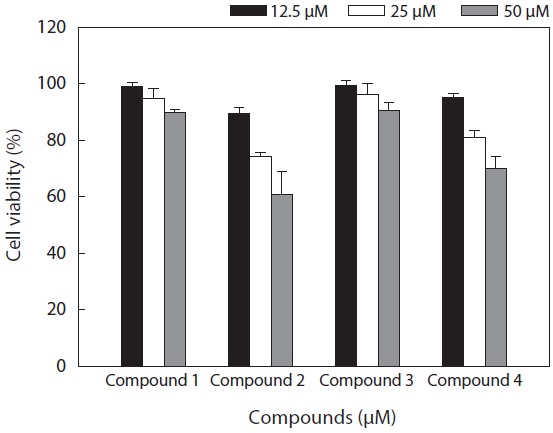
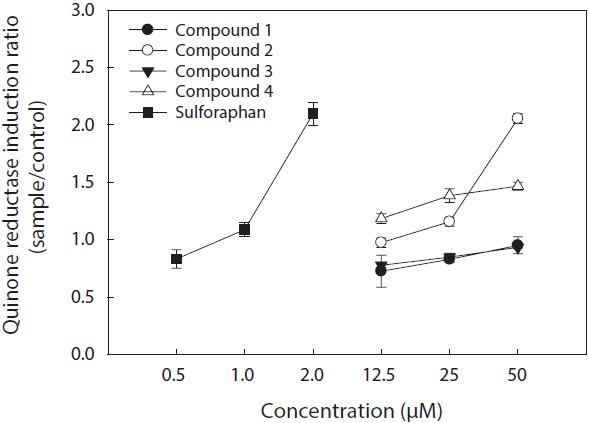
The cell viability and QR induction activity of the isolated compounds were also assessed as shown in Figs. 4 and 5, respectively. Of these, dioxinodehydroeckol and fucofuroeckol- A exhibited cytotoxicity on Hepa1c1c7 cells, whereas phloroglucinol (1) and dieckol (3) did not show cytotoxicity in the concentration range of 62.5-250 μg/mL.
Moreover, dioxinodehydroeckol (2) exhibited the most potent QR induction activities in Hepa1c1c7 cells, with a 2.05 ± 0.04 fold induction at 50 μM, compared to the DMSO treated control cells; however, it was lower than that of sulforaphane, which was used as a positive control. Fucofuroeckol-A (4) showed moderate QR induction activity with 1.46 ± 0.03-fold induction at 50 μM. Phloroglucinol (1) and dieckol (3) exerted no detectable QR induction activity in Hepa1c1c7 cells.
QR induction properties of phlorotannins were similar to those of representative QR active compounds derived from terrestrial organisms―quercetin, genistein, and resveratrol―which showed weak cytotoxicity on Hepa1c1c7 cells and exhibited potent QR induction activity (Yang and Liu, 2009). Although phlorotannins showed slightly lower QR induction activity than quercetin, genistein, and resveratrol, they were comparable (Yang and Liu, 2009). Sulforaphane, which was employed as a positive control, is known to have significant antitumor and anticarcinogenic enzyme induction effects (Zhang and Talalay, 1994; Zhang et al., 1994).
In conclusion, the EtOAc fraction from