Epoxy resins are well-known materials in the field of insulation systems for heavy electric equipments, due to their good mechanical and thermal properties and excellent electrical properties [1-3]. In order to improve these properties, many types of inorganic fillers such as silica (SiO2) [4], alumina (Al2O3) [5], mica [6], aluminum nitride (AlN) [7], and titanium dioxide (TiO2) [8], etc. have been incorporated into the epoxy resins. Messersmith and Giannelis [9] reported that the storage modulus of epoxy resin increased 58% at the glass transition region and increased 450% at the rubbery plateau region by loading of only 4 vol.% of clay. Mulhaupt et al. [10] demonstrated that the toughness and stiffness of epoxy resin were modified by adding nano-sized mica, bentonite or hectorite. T. Tanaka et al. [11] showed that epoxy/ layered silicate nanocomposite had much better insulation breakdown strength than that of the non-filled epoxy resin system in needle-plate electrodes geometry.We previously reported that the insulation breakdown strength of the epoxy/layered silicate nanocomposites was 33% higher than that of the system without nano-silicate [12].
However, when inorganic filler was incorporated into an epoxy resin, the viscosity became too high. Therefore, it was very difficult not only to disperse the fillers homogeneously and to remove bubbles from the epoxy/filler mixture but also to inject the viscous mixture into a mould during the curing process. Therefore, in order to decrease the viscosity, plasticizers, organic solvents and reactive diluents were individually introduced to the epoxy/filler composites so that the bubbles were easily removed from the composites after injecting into a mould.
However, the plasticizers disturbed the cure reaction of the epoxy system so that the crosslink density decreased. This caused a decrease in the electrical, mechanical, and thermal properties of the epoxy system. In addition, when organic solvent is used, it should be removed completely after mixing the epoxy, fillers, and organic solvent. If not, it can act as an impurity, negatively affecting the electrical, mechanical, and thermal properties of the epoxy composite. Reactive diluents were used in order to decrease the viscosity during mixing and they were reacted with epoxy system during the curing reaction. In this study, polyglycol and 1,4-butanediol diglycidyl ether (BDGE) were acted as reactive diluents. The former was acted as a flexibilizer and the latter was acted as a chain extender during curing reaction. The ac electrical breakdown strength and mechanical properties were then studied.
A commercial DGEBA (diglycidyl ether of bisphenol A) type epoxy resin, YD 128 (Kukdo Chem. Co.) was used. The equivalent weight was 184~190 and the viscosity was 11,500~13,500 cps at 25℃. The curing agent was Me-THPA (3- or 4-methyl-1,2,3,6-tetrahydrophthalic anhydride),the of which was HN-2200 (Hitachi Chem. Co.). It is widely used in the field of electrical insulation. The accelerator was BDMA (benzyl-dimethyl amine, Kukdo Chem. Co.). The reactive diluent as the flexibilizer employed was a polyglycol with the trade name of DY-040 (Ciba-Geigy Co.). Its molecular weight was about 7,000~10,000 and its viscosity was 60~90 cps at 25℃. Another reactive diluent as an aliphatic epoxy resin was purchased from Kukdo Chem. Co. with the trade name of BDGE. Its equivalent weight was 120~140 and its viscosity was 15~30 cps at 25℃.
DGEBA (100g), THPA (92 g), and reactive diluent (10 g) were well-mixed with a high-speed agitator at 5,000 rpm for 10 min, and BDMA (1 g) was mixed with the agitator for 3 min. The mixture was then poured into specimen molds for electrical insulation breakdown and mechanical tests and was then degased using a vacuum oven. It was then cured at 120℃ for 2 hr and continually post-cured at 150℃ for 2 hr, and thencooled slowly at a rate of -0.5℃/min until room temperature to avoid internal stress.
Specimens for the insulation breakdown test were designed to be 1 mm thick with a 100 mm diameter. The specimen dimension for dynamic mechanical analysis (DMA) was 12.5×7.0×3.0 mm. Specimens for the tensile test were prepared under the recommendation of JIS B7502 and those for the flexural test were prepared under the recommendation of JIS B7507 at a size of 70×10×4 mm.
2.3 AC insulation breakdown test
Sphere to sphere electrodes were arranged to have a 1 mm insulation thickness to measure the ac insulation breakdown strength. The electrodes were made of copper and their diameters were 7.40 mm. The specimen and electrodes were then dipped into an insulating oil of 30℃ and a high voltage (HV) was applied by an AC Endurance Voltage Tester (Haefely, Germany) at an increasing speed of 1 kV/s until the electrical insulation breakdown℃curred. The HV generator was specified to be controlled at the frequency of 60 Hz with the maximum voltage of 400 kV. The secondary maximum current was 1,000 mA with a system of 400 kVA. The voltage, current, and frequency data were automatically collected every 5 seconds, and all insulation breakdown strength data were estimated by Weibull statistical analysis.
Dynamic mechanical analysis (DMA2980, TA Instrument Ltd.) was carried out in a shear mode at the frequency of 1.0 Hz, a heating rate of 10℃/min, and an air purge rate of 110 ml/min.
Flexural and tensile tests were carried out using a universal testing machine (SHM-C-500, Shamhan Tech, Korea). The flexural test was carried out using the three point bending method. The span length was 50 mm and the crosshead speed was 10 mm/min at 23℃ and 50% relative humidity. The tensile test was performed at a crosshead speed of 10 mm/min at the same temperature and humidity as those in the flexural test.
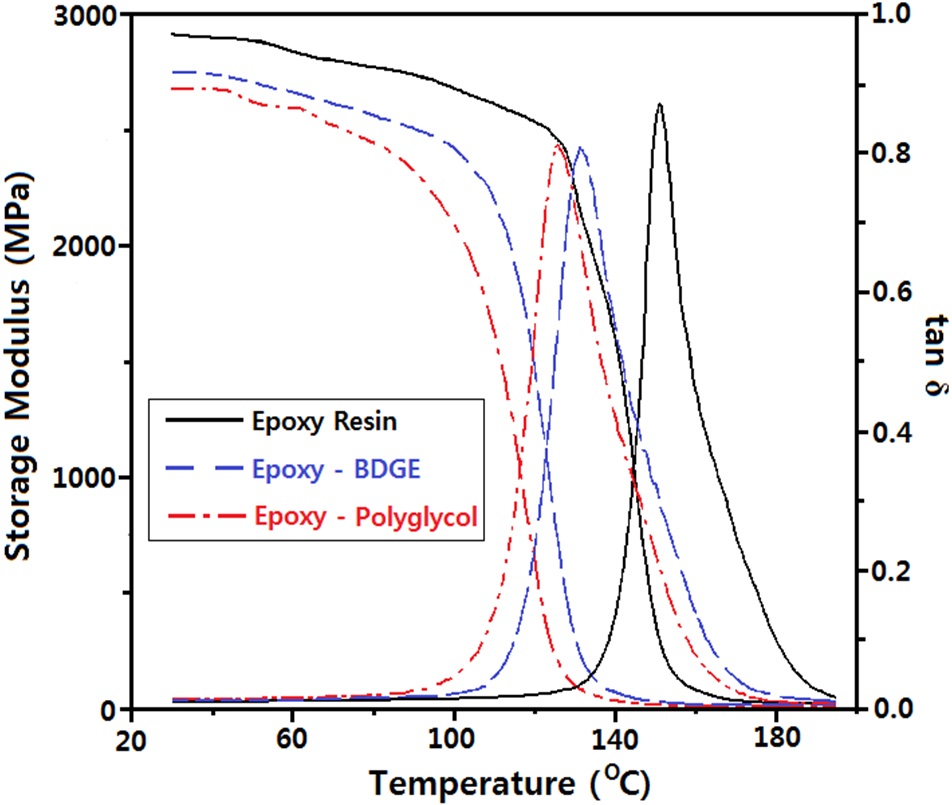
Two reactive diluents were used: one was a polyol type which could react with the epoxide group and the other was an aliphatic epoxy resin which could react with a curing agent. In order to study the effect of the diluents on the glass transition temperature (Tg) of the epoxy system, DMA analysis was carried out and is shown in Fig. 1. The storage modulus (G') of the epoxy system without diluent at 40℃ (at the glass region) was 2,910 MPa and the value for the system with BDGE or polyglycol was 2,745 MPa or 2,722 MPa, respectively. As the atmosphere temperature increased, the G' value decreased and an abrupt decrease was observed at around the glass transition temperature. As the atmosphere temperature increased, the loss modulus (G") abruptly increased at the glass transition, which was displayed in the form of tan δ = G"/G'. Therefore, Tg was estimated from the peak temperature of tan δ and is listed in Table 1.
The Tg value for the epoxy system without diluent was 152.0℃ and that for the system with BDGE or polyglycol was 131.8℃ or 126.1℃, respectively. In general, the Tg of polymers was affected by the polymer structure. Especially, Tg is directly proportional to the crosslink structure of the polymer matrix [12]. current study, the decreasing Tg indicated that the crosslink structure of the epoxy resin was reduced. This was because the aliphatic BDGE or the polyglycol wasacted as a chain extender so that the flexible chain length between the two crosslink points increased. Generally, a lower Tg should lead to a lower ac insulation breakdown strength in an epoxy system. However, in this study, the breakdown strength increased slightly in the epoxy-polyglycol system or there was no change in the strength of the epoxy- BDGE system as shown in Fig. 2.
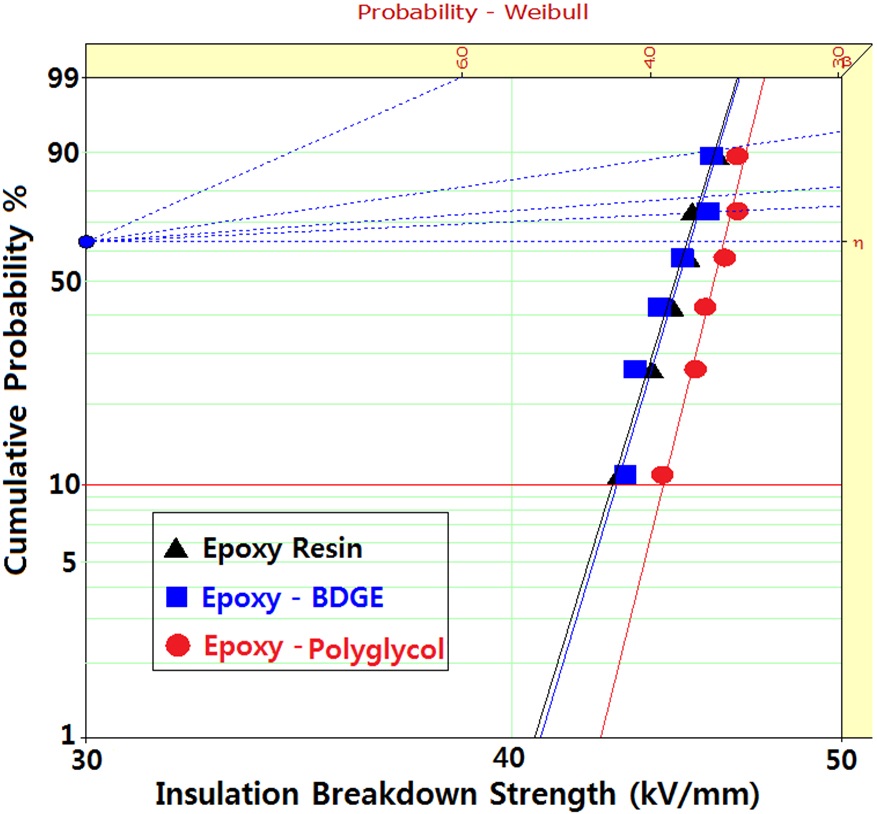
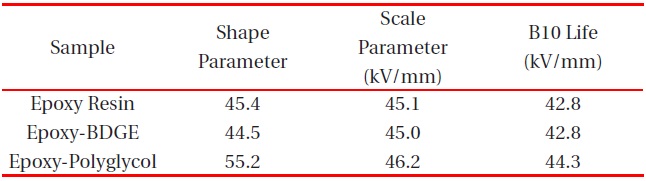
Figure 2 shows Weibull plots of ac electrical breakdown strength for the epoxy systems with and without reactive diluents. The specimen thickness was 1 mm, and the parameters such as shape and scale parameters, and the B10 value were obtained from the Weibull plots and are listed in Table 2. Here, the shape parameter could be obtained from the slope, indicating that the data distribution and the scale parameter represented the ac electrical breakdown strength by which 63.2% of the cumulative probability was expected to fail. The B10 value referred to the ac electrical breakdown strength at which 10% would fail (90% would survive) under a given electrical stress. The statistical analysis of the epoxy resin without a diluent showed that the scale parameter was 45.1 kV/mm with the shape parameter of 45.4. As the polyglycol was added to the epoxy system, the scale parameter became slightly higher and the shape parameter was ca. 10 higher. The values for the scale and shape parameters for the epoxy system with BDGE were almost the same as the epoxy system without the diluent. When examining the decreasing crosslink density and the scale parameters of Table 2, it can be seen that the negative effect of crosslink density on the electrical
[Table 1.] Effect of diluents on the glass transition temperature obtained from Fig. 1.

Effect of diluents on the glass transition temperature obtained from Fig. 1.
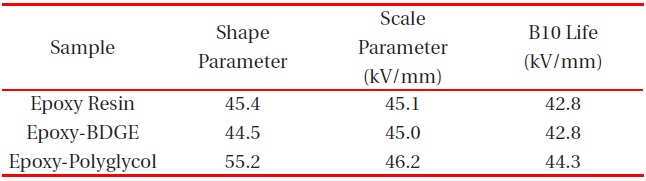
Weibull parameters for insulation breakdown strength in various epoxy systems at 30℃ obtained from Fig. 2.
breakdown strength was compensated by the positive effect of the diluents. In addition, the higher value of the shape parameter indicated the homogeneous composition of the reactants, leading to a low degree of dispersion. Therefore, if the polyglycol were used as a reactive diluent in an epoxy/inorganic filler system, it could be used in order to decrease the viscosity of the epoxy system.
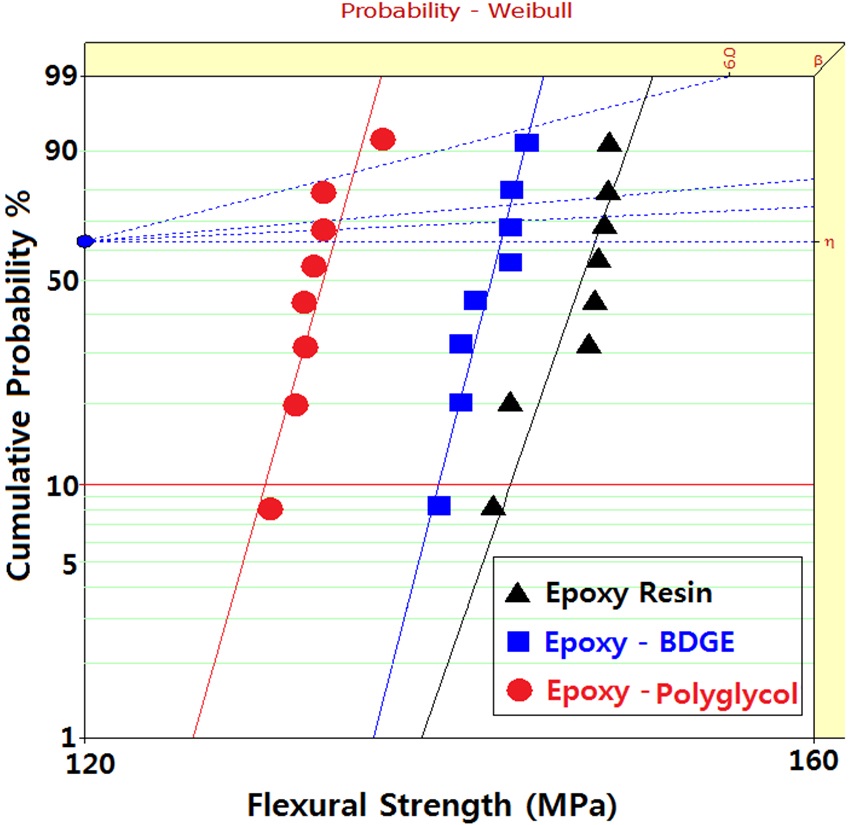
Figure 3 shows Weibull statistical analyses of flexural strength for epoxy systems with and without reactive diluents, and the
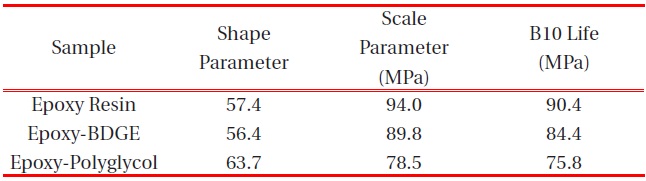
[Table 3.] Weibull parameters for flexural strength in various epoxy systems obtained from Fig. 3.
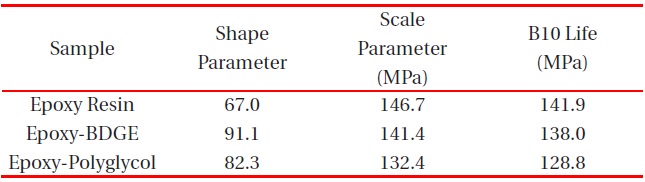
Weibull parameters for flexural strength in various epoxy systems obtained from Fig. 3.
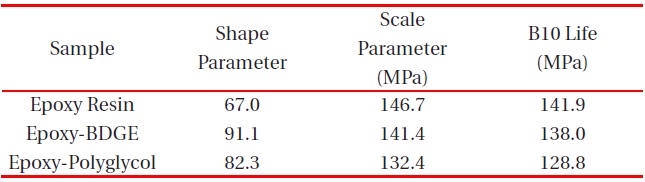
[Table 4.] Weibull parameters for tensile strength in various epoxy systems obtained from Fig. 4.
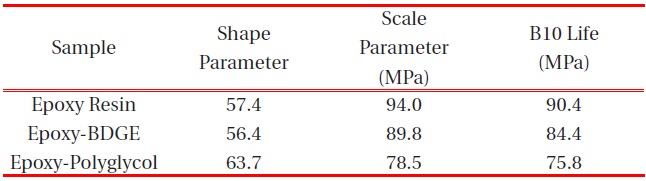
Weibull parameters for tensile strength in various epoxy systems obtained from Fig. 4.
parameters such as shape and scale parameters, and B10 value were obtained from the Weibull plots and are listed in Table 3. The statistical analysis showed that the scale parameter of the epoxy resin without reactive diluent was 146.7 MPa with the shape parameter of 67.0, and that of the epoxy system with polyglycol was 132.4 MPa with the shape parameter of 82.3. Moreover, the scale parameter of the epoxy system with BDGE was 141.4 MPa with the shape parameter of 91.1. As predicted, the flexural strength decreased when the reactive diluents were added. This meant that the reactive diluents acted as flexibilizers in the epoxy systems and some of the reactive diluents withstandcuring reaction so that the diluent monomers disturbed the cure reaction between the epoxide group of the epoxy resin and the carboxyl group of the curing agent. Therefore, the molecular weight between the two crosslink points increased and many end-chains were generated. These results caused easy mobility of the epoxy chains at lower temperature as the surrounding temperature increased. Therefore, Tg decreased by the addition of reactive diluents.
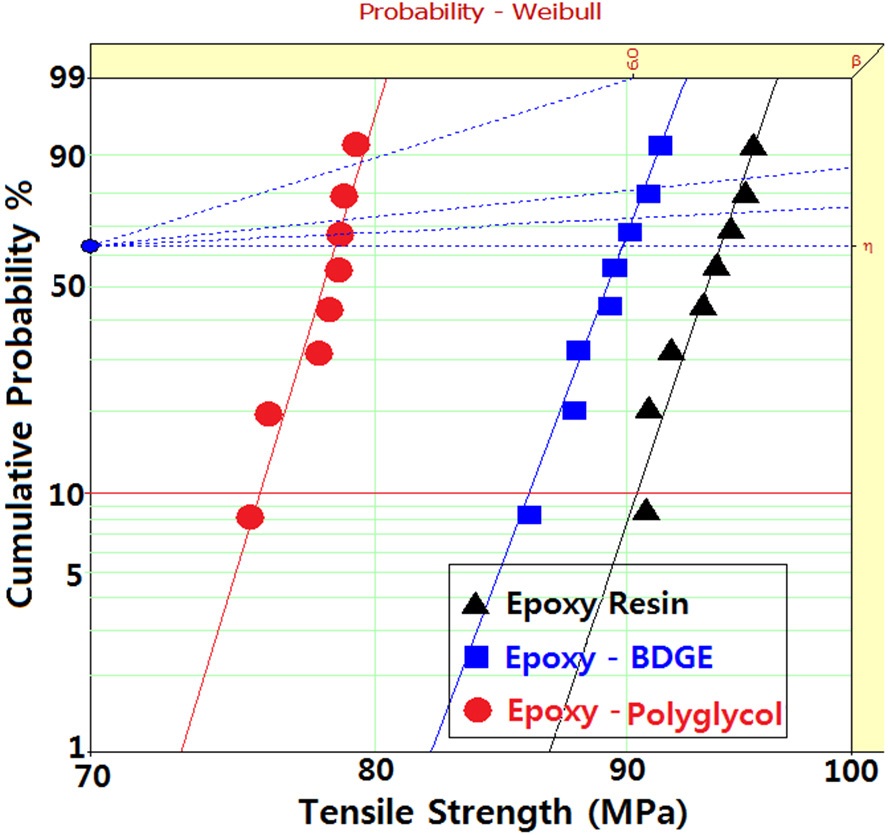
According to the addition of the reactive diluents, the same tendency was observed in the tensile strength as shown in Fig. 4 and the Weibull parameters obtained from Figure 4 are listed in Table 4. The scale parameter of the epoxy system without reactive diluent was 94.0 MPa with the shape parameter of 57.4, and as the BDGE was added to the epoxy system, it was 89.8 MPae parameter of the epoxy-polyglycol was 78.5 MPa, which was ca. 17% lower than that of the epoxy system without diluent. The shape parameter of the epoxy-polyglycol was 63.7, which was ca. 11% lower than that of the epoxy system without diluent.
The effect of reactive diluents on the ac electrical insulation breakdown strength and flexural and tensile properties was studied. All the data were estimated using Weibull statistical analysis. In the ac electrical insulation breakdown strength, the scale parameter of the epoxy system without reactive diluents was 45.1 kV/mm and its shape parameter was 45.4. As the polyglycol was added to the epoxy system, the scale parameter slightly increased and the shape parameter was ca. %. The higher value of the shape parameter meant that the polyglycol could be used as a reactive diluent. In the flexural strength, the scale parameter of the epoxy resin without reactive diluent was 146.7 MPa, while that of the epoxy system with polyglycol or BDGE was 132.4 MPa or 141.4 MPa, respectively. Tensile strengths of the epoxy resin, epoxy-BDGE, and epoxy-polyglycol systems were 94.0 MPa, 89.8 MPa, and 78.5 MPa, respectively. These mechanical strengths meant the reactive diluents acted as flexibilizers.