ZnS-based phosphors have been extensively studied as commercial phosphor, because of their wide band gap of 3.7~3.8 eV, high efficiency and stability of crystals [1]. Also, ZnS-based phosphors with various dopants have been tried to obtain various color expression and luminance enhancement. [2,3]. Generally, Mn-doped ZnS is recognized as an efficient phosphor [4], so that it is widely explored for various light source applications, such as fluorescent lamps, Electroluminescence display, field emission displays (FEDs) and light emitting diode (LEDs) [5].
Recently, research for efficient color conversion phosphors for white light source has sparked renewed interest in photoluminescence behavior of doped ZnS phosphor [6]. However, the ZnS phosphors converted by light source have a limitation of color gamut and luminance efficiency. There are only a few reports dealing with the effect of dopants to improve the color converting capability in ZnS phosphors [7].
In this paper, we selected magnesium as a dopant for ZnS:Mn phosphor material, and synthesized various concentrations of magnesium dopant by the solid state reaction method [8]. We investigated the luminescence converting properties of various concentrations of magnesium-doped ZnS:Mn phorphors at a wide range of excitation wavelengths. Finally, we evaluated the effect of the color converting capability of ZnS:Mn,Mg phorphors with plasma blue light emission generated by BaMgAl10O17:Eu2+(BMA), which are commercial phosphor materials that are used in plasma display panels.
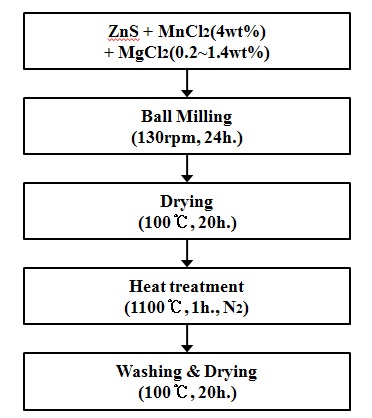
We synthesized various concentrations of manganese-doped ZnS:Mn phosphor by the solid state reaction method. The ZnS raw material (99.9%) was mixed with MnCl2·4H20 (1wt%) and MgCl2 in distilled water at room temperature. The amount of Mn dopant was fixed at 4 wt%, and the concentration of magnesium in ZnS was varied from 0.2 to 1.4 wt%. A ball-milling process was carried out for 24 hours, and then firing was conducted at 1,100℃ for 1 hour. The prepared ZnS powder was washed several times by distilled water and ethanol. Finally, the powders were thoroughly dried at 100℃ for 24 hrs. Figure 1 shows the experimental detail conditions for preparing ZnS:Mn,Mg phosphors. In order to evaluate the luminance properties of phosphor powder, it was
mixed with ethyl cellulose binder, and coated on a glass substrate by the screen printing method. We analyzed the ZnS:Mn,Mg phosphor by X-ray diffraction (XRD; D/MAX-220,Rigaku), scanning electron microscope (SEM; S-4700, Hitachi), and photoluminescence spectra (PL; FS-2, Scinco).
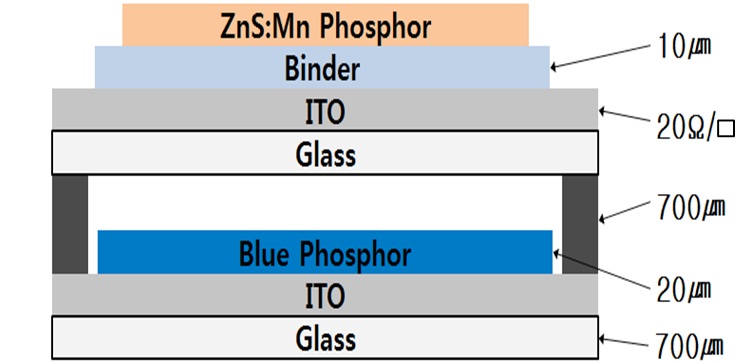
To investigate the luminescence properties of the magnesiumdoped ZnS:Mn phosphor excited by plasma blue light source, we applied various concentrations of magnesium-doped ZnS:Mn phosphor to the plasma luminescence device shown in Fig. 2. The front plate consisted of a magnesium-doped ZnS:Mn phosphor layer with various concentrations of magnesium dopant on the binder layer, which was adapted to improve adhesion between phosphor and ITO glass. The rear plate was printed by commercial blue phosphor onto ITO glass. After the blue phosphor layer was formed, the front plate and rear plate were sealed completely whit a gap of 700 um. The processed device was filled with Ne-Xe (5%) discharge gas with pressure of 150 Torr. [9,10].
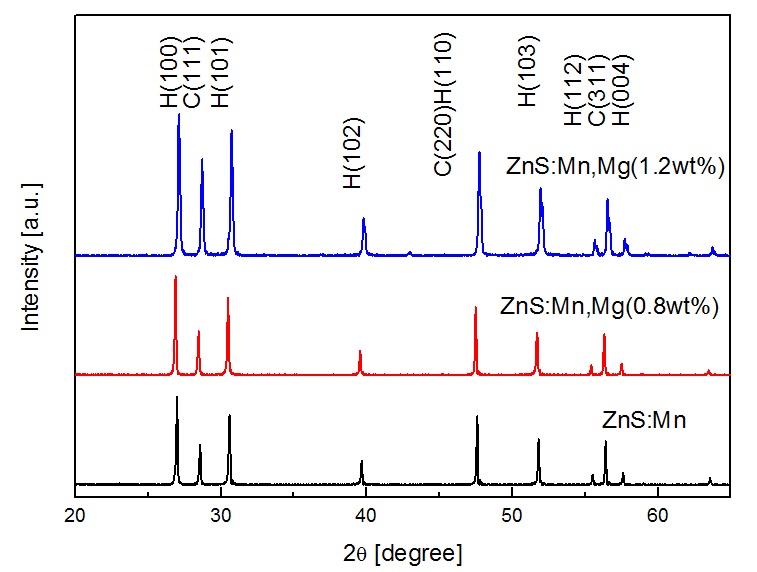
Figure 3 shows an XRD pattern of the ZnS:Mn phosphor with various magnesium dopant levels. As we can see, the two main peaks (100), (101) clearly indicate the formation of hexagonal wurtzite structure. All the ZnS:Mn phosphors, regardless of the magnesium dopant, were well crystallized, and show typical ZnS: Mn peaks. As the Mg concentration was increasd up to 1.2 wt%, the XRD intensity of ZnS:Mn showed stronger and sharper.
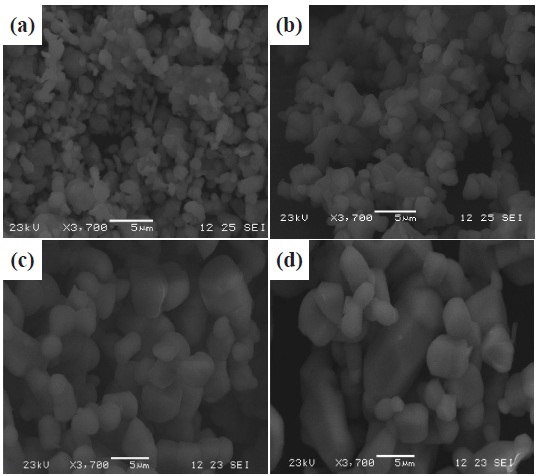
The SEM images in Fig. 4 show the ZnS:Mn phosphor with various levels of magnesium dopant. The sizes of ZnS:Mn powder increased, and the surfaces of powder became smoother with increasing Mg dopant concentration.
The size of particles measured by the SEM photograph was under 1 um at the condition of pure ZnS, and 3~5 μm at 1.2 wt% of Mg. Obviously, the size of ZnS powder is increased with Mg concentration, It is known that the activation energy of grain growth is reduced with the creation of Zn vacancy, by adding MgCl2 as the flux during the synthesis of phosphors. This implies that the use of MgCl2 as the flux results in an improvement in the growth and smooth morphologies of the ZnS Mn phosphor powders. [11].
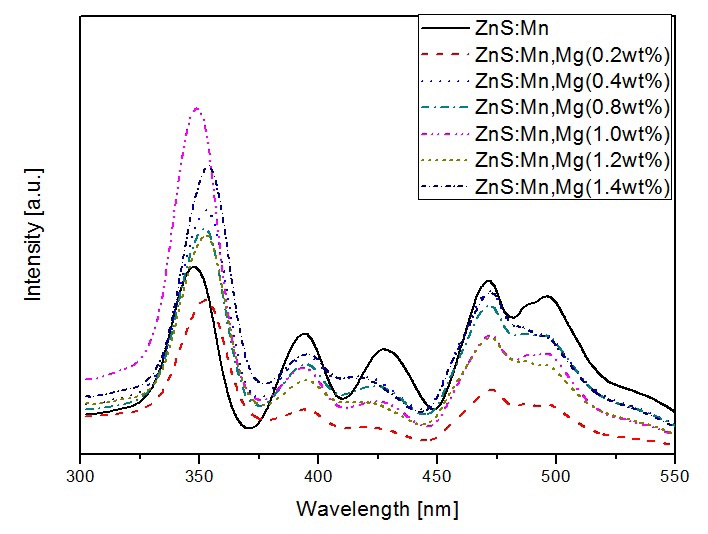
Figure 5 shows photoluminescence excitation spectra of ZnS:Mn phosphor with various magnesium dopant levels from 300 nm to 550 nm wavelength. We observed five absorption bands of Mn2+ around 350, 390, 425, 470, and 500 nm [4]. As the concentration of magnesium increased, the photoluminescence spectra intensity that could be distinguished increased at corresponding peak wavelengths of 350 nm. The highest photoluminescence excitation intensity of the phosphors was observed at an Mg concentration of 1.0 wt. Also, small shifts to the shorter wavelengths in the spectral feature are seen with addition of magnesium contents. We consider this progressive shift to be affected by lowering of the band gap energy by Mg dopant, which is related to the formation of additional energy levels [12].
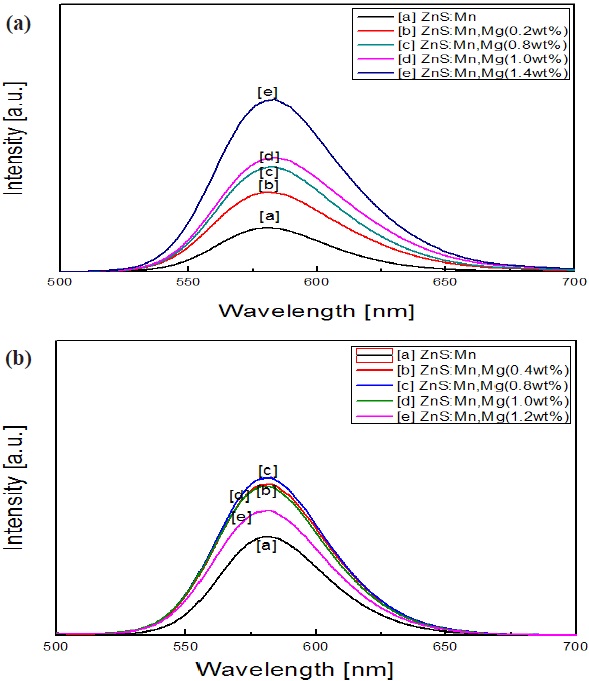
The photoluminescence spectra of magnesium-doped ZnS:Mn phosphors under 365 nm and 450 nm excitation wavelength are shown in Figures 6 (a) and (b), respectively. The PL emission spectra of ZnS:Mn and magnesium-doped ZnS:Mn exhibit only one band with corresponding peak wavelengths of 580 nm under excitation of 365 nm and 450 nm. The emission of 580 nm in ZnS:Mn phosphors was associated with 4T1→6A1 transition of Mn2+ ions. The PL intensity of Mg-doped ZnS:Mn appeared clearly higher than the intensity of ZnS:Mn without magnesium dopants. Also, we can see the gradual increase of PL intensity in the case of 365 nm excitation from 0.2 to 1.4 wt% of Mg dopant
level, whereas PL under 450 um shows that the saturation of PL intensity at 0.8 wt% implies that the PL intensity is closely related to powder size variation. This is caused by the amount of Mg content and the high intensity excitation energy of 365 nm rather than 450 um, which the emission of luminance transition energy suggests is formed by the impurity of Mg dopants [13].
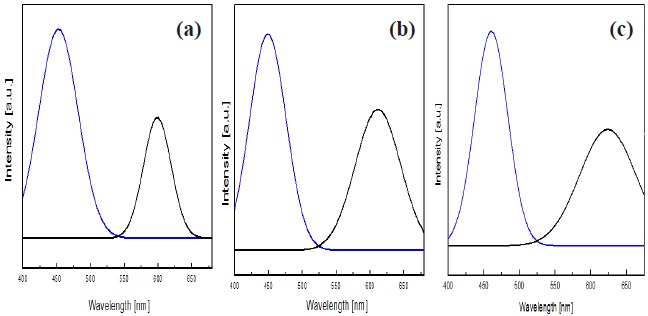
Figure 7 shows luminescence spectra of plasma blue light source and ZnS phosphors excited by a plasma luminescence device. As we introduced in the experimental, the plasma light source device consists of blue phosphor on organic binder with external electrodes. The luminance characteristics were evaluated at 220 V, duty 20% frequency 40 kHz condition, which shows 50 cd/m2 of luminance on the plasma device [14].
The white emitting light source was realized by ZnS:Mn and ZnS:Mn,Mg phosphors with blue light source, and we can see the luminance spectra of combined blue light source and yellowish ZnS phosphors. On increasing the magnesium content, the luminance intensity at 580 nm becomes higher and wider up to 0.8 wt%,
and saturated at 1.0 wt% of Mg content. The luminescence spectra of magnesium-doped ZnS:Mn in wavelength of 580 nm shifted to wavelengths of approximately 600 nm.
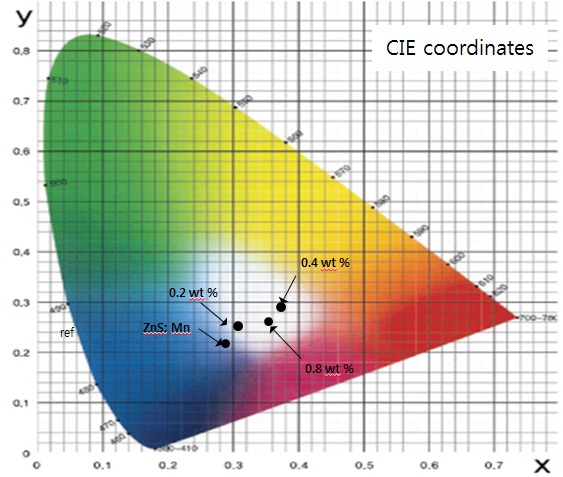
Figure 8 shows CIE coordinates of magnesium-doped ZnS:Mn phosphors excited by plasma blue light source. On increasing the Mg up to 0.8 wt%, the color coordinate shifted from CIE x=0.3044 to CIE x=0.3616. The measurement of CIE coordinates of ZnS:Mn, Mg 0.2, 0.8 wt% phosphors showed them to be x=0.3077, y= 0.2457 and x=0.3616, y=0.2626 respectively. These results showed enhanced 580~600 nm PL intensity by adding magnesium, and the shift of CIE coordinates of the ZnS:Mn, Mg phosphors indicated that Mg addition changes whitish color from cold white to warm white emission.
In summary, we synthesized various concentrations of magnesium- doped ZnS:Mn phosphor by the solid state reaction method. The PL intensity of Mg-doped ZnS:Mn appeared clearly higher than the intensity of ZnS:Mn without magnesium dopants. Also, we can see the gradual increase of PL intensity in the case of 365 nm excitation from 0.2 to 1.4 wt% of Mg dopant level, whereas PL under 450 nm shows the saturation of PL intensity at 0.8 wt%. The synthesized ZnS:Mn, Mg phosphors were applied to blue plasma luminescence devices, in order to investigate the color conversion properties of ZnS:Mn, Mg to make white light sources. Successively, the white emitting light source was realized by ZnS:Mn and ZnS:Mn,Mg phosphors with blue light source. The CIE coordinate shifted from x=0.3044 at 0.2 wt%, to CIE x=0.3616 at 0.8 wt%. From the luminance spectra generated by ZnS:Mn,Mg phosphors with blue plasma light source, we found enhanced 580~600 nm PL intensity by adding magnesium, and the shift of CIE coordinates of the ZnS:Mn, Mg phosphors. It is obvious that Mg addition into ZnS:Mn phosphor can be a effective way of changing whitish color, from cold white to warm white emission.