Larvae of the hornless Australian burrowing mayfly genus
Demoulin (1955) established the genus
The purpose of this study is to revise members of the genus
Type and voucher specimens and additional fresh material of
In the descriptions, eye size is measured by the ratio (B/D) of the shortest distance between compound eyes to the longest dorsal diameter of a compound eye. Gill expansion rate is defined by the ratio of the vertical height of apicomedial expansion of gill lobe (measured by the direct line of the height from the base of apical filament of gill to the apex of expansion) against gill lobe length from the base of gill to the base of apical filament of gill. Other terminology, measurement and general methods follow those of Bae and McCaf-ferty (1991), Dean (1999), and Bae et al. (2004). Abbreviations used in this study follow those of Bae et al. (2004): male imago (MI), female imago (FI), male subimago (MS), female subimago (FS), larva (L) and egg (E); New South Wales (NSW), Queensland (QLD), Victoria (VIC) and South Australia (SA).
Order Ephemeroptera
Family Leptophlebiidae
Genus Ulmerophlebia Demoulin, 1955
Type species.
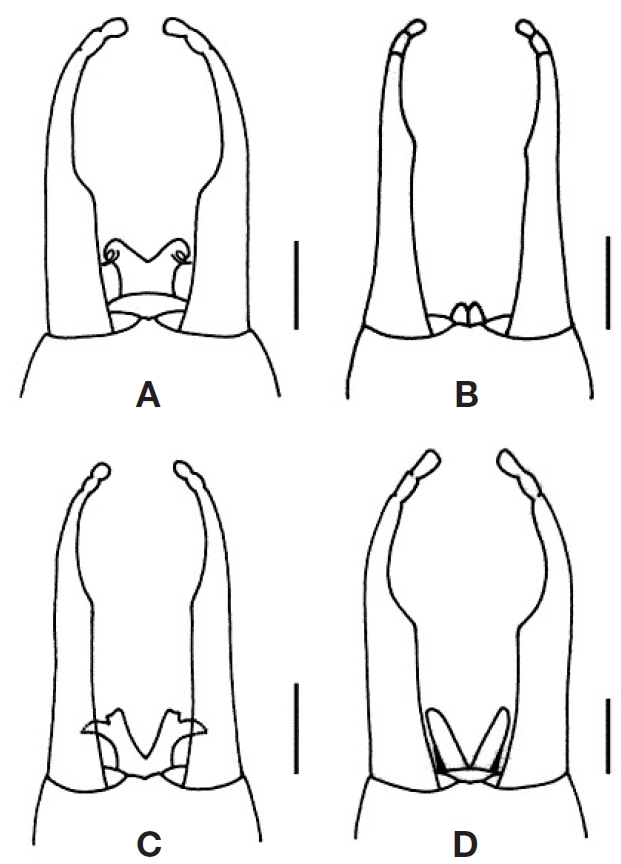
Redescription. Imago: Male body length 6.9-8.7 mm, caudal filaments 7.5-11.0 mm; female body length 6.3-8.6 mm, caudal filaments 10.6-10.7 mm. General body colour light yellow with dark purplish brown markings. Male compound eyes dorsally oval and dome-shaped, with anterior margin circular and posterior margin slightly attenuating from lateral view (‘bicycle helmet-shaped’), contiguous (B/D=0-0.06); basal compound eyes anteroventrally pronounced. Female compound eyes widely separated (B/D=3.11-4.91). Forewings transparent, often with dark purplish brown markings in basal, central and apical areas, length 6.6-8.5 mm, width 2.2-3.2 mm; longitudinal veins light brown to dark purplish brown; crossveins light brown to dark purplish brown (basally and anteriorly located crossveins often darker and more heavily infuscated); crossveins C-Sc 12-19; MP2 basally connected to MP1 and CuA; ICu1 basally connected to CuA and CuP (angle between ICu1 and crossvein ICu1-CuP larger than angle between ICu1 and crossvein ICu1-CuA); stigmatic area not anastomosed. Hindwings transparent, length 1.3-1.9 mm, width 0.7-1.0 mm; crossveins C-Sc 4-10, concentrated apically; costal margin round with weakly developed costal projection at 1/3 basally to midlength. Male foretarsi > foretibiae > forefemora; foretarsal segment 2 ≥ 3>4 > 5 > 1. Female foretibiae > forefemora>foretarsi. Claws dissimilar. Penes Y-shaped or V-shaped, normally developed or rudimentary; forceps 3-segmented; forceps basal segment parallel-sided, narrowing at 1/2-2/3 length; forcep segments 2 and 3 often indistinctly demarcated. Caudal filaments light yellow, often with purplish brown to dark brown stripes and annulations at joints.
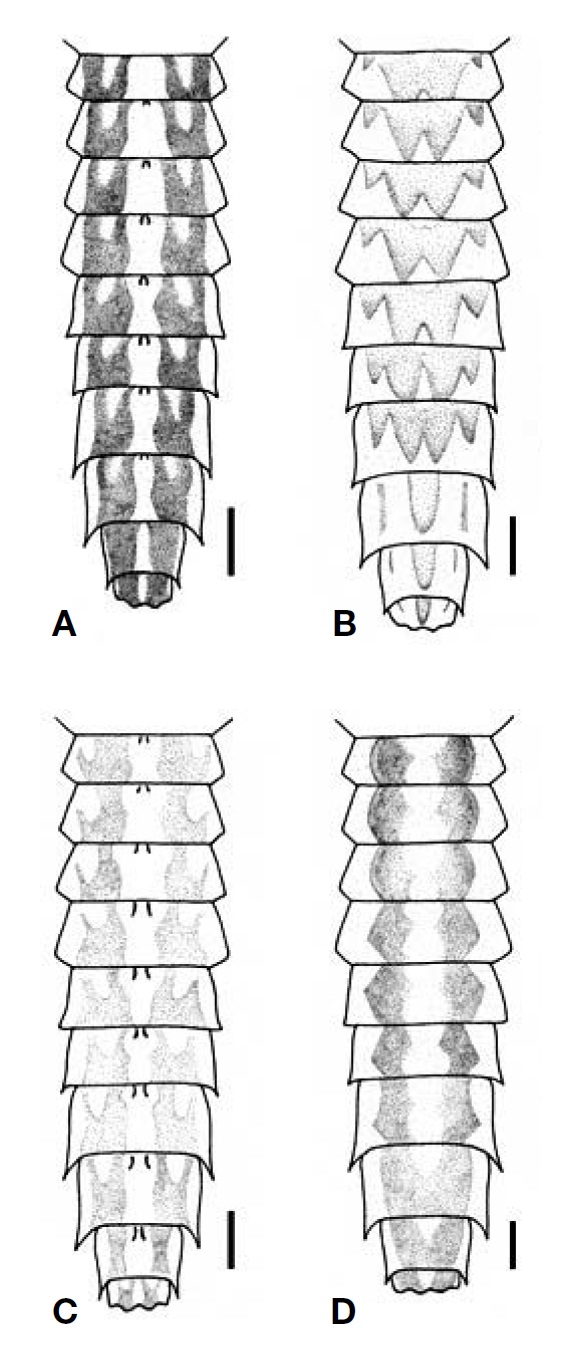
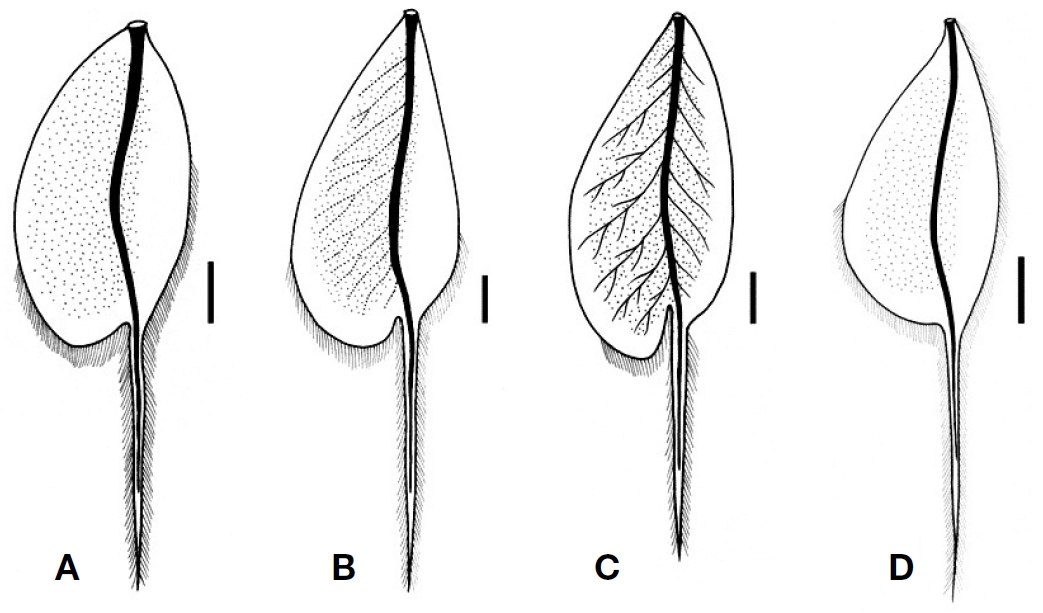
Larva: Male body length 6.2-9.8 mm; caudal filaments 5.5 -10.0 mm. Female body length 6.8-11.2 mm; caudal filaments 5.5-13.2 mm. Body surface glassy and relatively less setose. General body colour light yellow to light brown with dark purplish brown markings. Head without cephalic tusks. Clypeus greatly developed (length 0.38-0.45 mm), with pronounced sublateral tubercles, with fields of hair-like setae subapicolaterally and subbasolaterally. Antennae with whorls of hair-like setae at each segment. Labrum slightly narrower than clypeus, slightly wider distally; dorsal surface with basal and subapical hair-like setal fringes; subapical setal fringe laterally longer and not densely arranged; anterior and lateral margins with dense hair-like setal row; anterior margin slightly concave, with prominent median tubercle, with three pairs of rudimentary submedian denticles; ventral surface with dense hair-like setal field along anterior margin, with row of 15-18 stout setae on subanterior margins, with paired fields of 10-15 hair-like setae on central areas opposite to median line. Mandible dorsolateral margins with very long hair-like setal row; ventral surface without setal row; inner incisors slightly smaller to as large as outer incisors; incisors with 2 -3 apical teeth and 0-4 lateral denticles; prostheca rudimentary, with well developed fringe. Hypopharynx superlinguae laterally curved and pointed apically. Maxillae with dense hair-like setal field, mixed with row of comb-like setae almost entirely on galealacinial crown, without medioapical comblike seta, with row of dense hair-like setae on medial margin; maxillary palp 3-segmented; segment 3 indistinctly demarcated from segment 2, with moderately developed setal field along outer margin. Labial glossae dorsoventrally elongated and ventrally stalked, with dense hair-like setae; paraglossae with dense hair-like setal field dorsoapically; labial palp 3- segmented; segment 3 indistinctly demarcated from segment 2, pointed apically, with dense hair-like setal field along outer margin, with stout setal row along inner margin. Pronotum anterolateral margins round; lateral margins with row of hair-like setae. Forefemora ≥ foretibiae > foretarsi > foreclaws; forefemora with a few hair-like setae on dorsal and ventral surfaces, with hair-like setal field along posterior margin (anterior margin with few hair-like setae); foretibiae with relatively sparse hair-like setae (filtering setae) dorsally and along outer margin (inner margin with few hair-like setae), with stout setal field (raking setae) ca. 5/6 apically on inner margin; foreclaws with row of tiny teeth basally. Abdominal terga light yellow to light brown, mostly with broad submedian dark brown stripes, with or without long hair-like setal field along median line, with hair-like setal row along lateral margins; abdominal segment VI-IX with moderately to well developed posterolateral projections. Abdominal sterna bare. Gills on abdominal segment I-VII, double; both lamellae with indistinct to distinct tracheae, with single apical filament, clothed with fine marginal seta, with weakly to strongly developed apicomedial expansion. Caudal filament with whorls of setae.
Egg: Egg oval; long axis ca. 1.5× length of short axis. Colour light yellow in nature, white in alcohol. Egg surface with knob-terminated coiled threads nearly evenly distributed throughout egg surface, with numerous tiny granules throughout egg surface. Polar caps absent.
Diagnosis. Although no fundamental adult key characters are found to distinguish
Distribution. QLD, NSW, VIC and SA.
Remarks. Dean (1999) considered a possible inclusion of
The suggested common name of this genus is the ‘hornless Australian burrowing mayflies’.
Ulmerophlebia annulata (Harker) (Figs. 1A, 2A, 3A)
Ulmerophlebia AV2: Dean, 1999: 75 (L key).
Material examined. NSW: 3 MI, Jindabyne, 21, 22 Feb 1969, Neboiss A [MV]; 6 FI, Wallagaraugh R at Princess Hwy Br, 29 Jan 1975, Neboiss A [MV]; 3 L, Genoa R at Cann Valley Hwy, 15 Nov 1994, Dean J [MV]; 8 L, Upper Kangaroo R, 28 Oct 1972, Dean J [MV]. VIC: 2 MI, Traralgon Ck, Koornalla, 26 Feb 1974 [MV]; 1 MI, Tennyson Ck at 5 km NW of Buldah, 149° 07′E, 37° 14′S, 1-7 Jan 1982 [MV]; 3 MI, Genoa Ck Falls, 3 km W of Genoa, 28 Mar 1974, Neboiss A [MV]; 1 MI, Clunes, 6 Jan 1956, Neboiss A [MV]; 1 MI, Coliban R at 6 km SW of Kyneton, 18 Feb 1973, Neboiss A [MV]; 1 FI, Bells Clearing, 6 km S of Aberielby, 10 Feb 1977, Calder AA [MV]; 4 FI, Mitchell R, 26, 29 Apr 1975, at light [MV]; 1 FI, Diamond Ck at 7 km SE of Gembrook, 31 Jan 1976, Wells A, Neboiss A [MV]; 1 FI, North Noorinbee, 20 Mar 1977, Neboiss A [MV]; 1 MI, Upper Gellibrand R, Stevensons Falls, 20 Jan 1982, Neboiss A, Wells A [MV]; 2 MI, 2 FI, 1 MS, Gellibrand R at Bryant Ck Jct, 24, 26 Jan 1982, Neboiss A [MV]; 1 MI, 2 FI, Gellibrand R at Alpine Ck Jct, 28 Jan 1982, Neboiss A [MV]; 1 FI, Healesville, Koranderrk Reserve, 21 Jan 1976, at light, Rankin, Smith [MV]; 8 FI, Yarra R below Upper Yarra Dam, 28 Feb 1976, Neboiss A [MV]; 7 L, Yarra R at Reefton Rd, 17 Feb 1978, Dean J [MV]; 7 L, Yarra R at Hazlewood Rd, 17 Feb 1978, Dean J [MV]; 1 MI, 2 FI, 1 FS, Tonghi Ck, 10 km SW of Cann R, 149° 06′E, 37° 37′S, 4 Feb 1987, at MV-light, Walker, McPhee [MV]; 2 MI, 1 FI, Cann R, 33 km N of Cann R, 149° 11′E, 37° 25′S, 5 Feb 1987, at MV-light, Walker, McPhee [MV]; 1 MI, 1 FI, Cann R, 14 Feb 1999, Finlay KJ [MU]; 1 FI (reared, with L exuvium), 1 FS (reared, with L exuvium), 10 L, 3 Mile Dam, 1 Dec 1997, Finlay KJ [MU]; 1 MI, Jackson Ck, 26 Oct 1997, Finlay KJ [MU]; 1 FI (reared, with L exuvium), Gulf stream, 16 Jan 1999, Finlay KJ [MU]; 1 FI (reared, with L exuvium), Smepthel Ck, 2 Mar 1997, Finlay KJ [MU]; 1 L (EPH-1845: ANVC), Loddon R at Glenluce- Malmsburg Rd, 22 Nov 1981, Dean J [MV]; 1 L (EPH- 1816: VVC), same data as EPH-1845 [MV]; 5 L, Rose R, 17 Mar 1999, Finlay KJ [MU]; 1 L, Lake Lilla, 29 Dec 1996, Finlay KJ [MU]; 1 L, Franklin, 11 Feb 1999, Finlay KJ [MU]; 1 L, Morwell, 4 Mar 1999, Finlay KJ [MU]; 3 MI (reared, with larval exuviae), 1 FI (reared, with larval exuvium), 6 L, Licola, Wellington R at 3 km u/s from Alpine NP entrance, 18 Mar 2001 (emerged 5 Jun 2001 at 18℃ ), Bae YJ, Govedich F, Bain B [MV, MU]; 1 L, Licola, Wellington R at 3 km u/s from Alpine NP entrance, 16 Apr 2001, Bae YJ, Lim KJ [MU].
Redescription. Male imago: Body length 7.2 mm. General body colour light yellow with dark purplish brown markings. Dorsal compound eyes dark orange in alcohol, length 0.92 mm, width 0.81 mm, height 0.44 mm, widely separated anteriorly and meeting posteriorly (B/D=0); basal compound eyes dark grey, length 0.59 mm, height 0.33 mm. Antennae 0.75 mm, white; segment 2 dark purplish brown. Thorax colour golden yellow with irregular dark brown markings; pronotum posteromedially greatly concave, medially and posteromarginally black, submedially brown, laterally white; mesonotum golden yellow, posterolaterally light brown with dark brown posteromedian hump; pleura and sterna with various irregular dark brown markings and spots; metanotum dark brown. Forewings hyaline, length 7.9 mm, width 2.6 mm;
longitudinal veins stained light brownish; crossveins brown to dark purplish brown and basally and anteriorly thicker in colour; crossveins C-Sc 19, infuscated; crossveins Sc-R1 16, basal 1/2 infuscated; crossveins R1-R2 14, slightly infuscated; stigmatic area milky. Hindwings hyaline, length 1.5 mm, width 1.0 mm; longitudinal veins and crossveins (except crossveins C-Sc and Sc-R1) hyaline; crossveins C-Sc 6, dark purplish brown, apically concentrated; crossveins Sc-R1 5, dark purplish brown; costal area round with indistinct costal projection at 1/3 basally; Rs 0.33 mm; R1 0.70 mm; MPs 0.20 mm; MP1 0.88 mm. Legs light yellow with joints dark brown; dorsal and ventral forefemora with purplish brown markings basally, at mid-length and apically; forefemora 1.45 mm, foretibiae 2.50 mm, foretalsal segment 1 0.10 mm, segment 2 0.68 mm, segment 3 0.70 mm, segment 4 0.55 mm, segment 5 0.25 mm and foreclaws 0.15 mm; claws dark purplish brown. Midlegs and hindlegs colour and markings similar to forelegs. Abdominal terga dark purplish brown (terga VII-X purplish brown) with broad longitudinal median white stripe (each segment including anteromedian dark spot) and anterosublateral and lateral white areas, with anterolateral dark purplish brown spots as seen in larval abdomen. Sterna with dark purplish brown longitudinal stripe (stripe in each segment posteriorly somewhat broader). Penes (Fig. 1A) white, length 0.13 mm, Y-shaped, with apicolateral dark brown spines (spines ventrolaterally curved); forceps light brown, dark brown apicolaterally, white apicomedially; forceps segment 1 0.63 mm, segment 2 0.09 mm and segment 3 0.08 mm; forceps segment 1 broad basally and slender apically, with distinct constriction at midlength, arched dorsally at constriction; segments 2 and 3 fused. Caudal filaments light yellow, with dark and light brown alternating joints; cerci 8.8 mm; terminal filament 11.0 mm.
Female imago: Body length 7.3 mm. General body colour and markings similar to male. Compound eyes width 0.28 mm; distance between compound eyes 0.88mm(B/D=3.11). Forewings length 8.5 mm, width 2.7 mm; veins somewhat darker in colour than veins in male; venation similar to male. Hindwings length 1.5 mm, width 0.9 mm; veins somewhat stained light brownish; venation similar to male. Abdomen colour and markings similar to male. Caudal filaments similar to male; cerci 9.0 mm; terminal filament 10.7 mm.
Larva: Male body length 6.2 mm; caudal filaments 5.5 mm. Female body length 6.8-7.2 mm; caudal filaments 5.5-6.0 mm. Dorsal body colour light brown to brown with light yellow markings; ventral body and legs light yellow with brown to dark brown markings. Head light brown, length 1.10mm and width 1.45 mm, with white areas between lateral ocelli and compound eyes. Male compound eyes width 0.50 mm; distance between compound eyes 0.55 mm (B/D=1.10). Female compound eyes width 0.20 mm; distance between compound eyes 1.10 mm (B/D=5.50). Antennae white, length 3.3 mm; segment 2 light brown. Clypeus length 0.38 mm, basal width 0.69 mm, apical width 0.66 mm, with well developed sublateral projections, with ca. 8-10 hair-like setae subapicolaterally, with ca. 10-15 hair-like setae subbasolaterelly. Pronotum length 0.50 mm, width 1.50 mm, with median dark brown stripe; lateral margins white. Mesonotum laterally with dark brown irregular markings; forewingpads with small black spots basally, centrally and laterally. Pleura and sterna with various dark brown markings. Forefemora 1.15 mm, foretibiae 1.00 mm, foretarsi 0.43 mm and foreclaws 0.15 mm; coxae dorsally with hair-like setal field; trochanters anterodorsally with row of 5-10 hair-like setae. Abdominal terga with broad median light area and anterosublateral light areas with submedian dark spots (Fig. 2A). Gills apicomedial expansion moderately developed (gill 4 expansion rate ca. 0.08); lateral tracheae invisible (Fig. 3A).
Diagnosis. The male adult of
guished by the gills of which the apicomedial expansion is moderately developed and the lateral tracheae are invisible (Fig. 3A). Larval abdominal terga have broad median light areas and small dark brown submedian spots (Fig. 2A).
Distribution. NSW, VIC.
Remarks. Harker (1950) described the male and female adults of
Ulmerophlebia deani Bae & Finlay, n. sp. (Figs. 2B, 3B)
Type material. Holotype: Mature female larva (EPH-1260: ANVC), Australia, Southeastern Queensland, Stony Creek, Conondale Range, 26 Aug 1997, Dean J [MV]. Paratypes: 1 mature male larva (EPH-1437), Australia, Northern Queensland, Barron River at Goonarra Creek, 6 Nov 1995, Herbert B [MV]; 1 mature female larva (EPH-1089), Australia, Northern Queensland, Birthday Creek at Paluma, 11 Aug 1990, Benson L [MV].
Other material. QLD: 1 L, Birthday Ck nr Paluma, 29 Aug 1997, Cartwright D [MV].
Description. Imago and subimago: Unknown.
Larva (holotype): Female body length 9.0 mm; caudal filaments 9.8 mm [Male body length 7.0 mm; caudal filaments 5.6 mm]. General body colour light yellow with dark purplish brown markings. Head light brown, length 1.56 mm, width 1.90 mm, with dark brown areas at vertex and between lateral ocelli. Female compound eyes width 0.24 mm; distance between compound eyes 1.43 mm (B/D=5.96) [Male compound eyes width 0.56 mm; distance between compound eyes 0.40 mm (B/D=0.71)]. Antennae length 4.0 mm; 2nd segment dark brown. Clypeus greatly developed, length 0.52 mm, basal width 1.00 mm, apical width 0.84 mm, with well developed sublateral projections, with ca. 8-10 hair-like setae subapicolaterally, with ca. 4-6 hair-like setae subba-
solaterally. Labrum slightly narrower than clypeus, distally slightly wider (maximum length 0.24 mm; basal width 0.73 mm; distal maximum width 0.75 mm). Maxillary palp segment 1 0.48 mm, segment 2 0.32 mm, segment 3 0.16 mm. Labial palp segment 1 0.48 mm, segment 2 0.29 mm, segment 3 0.24 mm. Pronotum length 0.87 mm, width 1.90 mm, with submedian and sublateral dark brown irregular markings; lateral margins white. Mesonotum with submedian dark brown irregular markings and dots; forewingpads with small black spots basally, laterally and centrally. Sterna with various dark brown markings. Forelegs without distinct markings; forefemora 1.75 mm, foretibiae 1.59 mm, foretarsi 0.63 mm, foreclaws 0.24 mm; forefemora with a few hair-like setae on dorsal and ventral surfaces, with hair-like setal field along posterior margin (anterior margin with few hair-like setae); foretibiae with relatively sparse hair-like setae dorsally and along inner and outer margins, with stout setal field along inner margin 4/5 apically; foretarsi with hair-like setae covering dorsal and lateral surfaces; foreclaws apically dark brown. Midlegs and hindlegs without distinct markings. Abdominal terga with W-shaped broad median dark brown markings, without submedian dark spots, without long hairlike setal field along median line, with hair-like setal row along lateral margins; abdominal segment VI-IX with well developed posterolateral projections (Fig. 2B). Abdominal sterna bare, without markings. Gills apicomedial expansion moderately developed (gill 4 expansion rate ca. 0.10); lateral tracheae ambiguously visible (Fig. 3B). Caudal filaments white.
Diagnosis. The larva of
Etymology. This species is named after Mr. John Dean who recognized this species for the first time (Dean, 1999).
Distribution. QLD.
Remarks. The suggested common name is the ‘W-marked hornless burrowing mayfly.’
Ulmerophlebia minuta Bae & Campbell, n. sp. (Fig. 1B)
Type material. Holotype: Male imago (EPH-1080), Australia, Northern Queensland, Kuranda, c 360 m, 30 Mar 1976, Quick WNB [MV]. Paratypes: 10 female imagos (EPH- 1079), same data as Holotype [MV].
Other material. QLD: 25 FI, 1 MS, same locality as holotype, 21, 23 Jan 1975, 30 Mar 1976, 8, 21 Apr 1976, Quick WNB[MV].
Description. Male imago (holotype): Body length 6.9 mm. General body colour light yellow with dark purplish brown markings. Dorsal compound eyes orange in alcohol, length 0.95 mm, width 0.87 mm, height 0.56 mm, broadly meeting posteromedially (B/D=0); basal compound eyes black, length 0.64 mm, height 0.29 mm. Thorax colour orange with various dark brown markings; pronotum length 0.24 mm, width 0.71 mm, deeply concave posteromedially, with submedian and sublateral dark brown stripes; pleura and sterna with various irregular dark brown markings and spots including large vertical markings on mesopleura; metanotum dark brown. Forewings hyaline, length 6.9 mm, width 2.4 mm; longitudinal veins hyaline; crossveins dark purplish brown; crossveins C-Sc 16, strongly infuscated; crossveins Sc-R1 12, infuscated; crossveins R1-R2 11; crossveins in stigmatic area not anastomosed (basally located 3 crossveins in stigmatic area closely located each other and forming large dark purplish brown marking). Hindwings hyaline, length 1.3mm, width 0.8 mm; veins hyaline; crossveins C-Sc 5, apically concentrated; crossveins Sc-R1 5; costal area round with indistinct costal projection; Rs 0.40 mm; R1 0.56 mm; MPs 0.24 mm; MP1 0.71 mm. Legs light yellow with joints dark brown; dorsal forefemora with purplish brown markings basally, at mid-length and apically; forefemora 1.59 mm, foretibiae 2.22 mm, foretarsal segment 1 0.10 mm, segment 2 0.95 mm, segment 3 0.84 mm, segment 4 0.32 mm, segment 5 0.11 mm, foreclaws 0.24 mm. Abdomen colour white with dark brown markings; abdominal segment 1 entirely dark brown (forming unique dark brown ring-marking together with dark brown metanotum); terga with narrow dark brown stripes posteromarginally and anteromedially (forming T-shaped marking), with anterolateral and subanterolateral dark brown spots (anterolateral spots darker). Penes (Fig. 1B) rudimentary, light yellow, with joints light brown, length 0.05 mm, slightly visible from ventral view; forceps light yellow; forceps segment 1 0.64 mm, segment 2 0.08 mm, segment 3 0.06 mm; forceps segment 1 basally broad and apically slender with distinct constriction at 2/3 apically. Caudal filaments length 7.5 mm, light yellow with alternating broad and narrow dark brown markings.
Female imago: Body length 6.3-7.9 mm. General body colour and markings similar to male. Compound eyes width 0.29 mm; distance between compound eyes 1.03mm(B/D= 3.60). Forewings length 8.5 mm, width 2.9 mm; venation, veins colour and markings similar to male. Hindwings hyaline, length 1.6 mm, width 0.9 mm; veins hyaline; venation similar to male. Abdomen basally with unique dark brown ring-marking as in male; terga with elongated round dark brown submedian markings and with anterolateral and subanterolateral dark brown dots; terga VII-IX light purplish brown. Caudal filaments 10.6 mm, with alternating broad and narrow dark brown markings.
Larva: Unknown.
Diagnosis. The male adult of
Etymology. The specific name of this species refers to the ‘minute’ or rudimentary penes which characterize this species.
Distribution. QLD.
Remarks. The type specimens of this species were labeled as
Ulmerophlebia mjobergi (Ulmer) (Figs. 1C, 2C, 3C)
Material examined. QLD: 1 MI, 3 FI, Canal Ck, u/s Jct Eliot Ck, 142° 25′E, 11° 23′S, at UV light, 6 Feb 1992, Cartwright D, Wells A [MV]; 1 L, Eliot Ck, u/s Jct Canal Ck, 7 Feb 1992, Cartwright D, Wells A [MV]; 15 MS, 4 FS, Cape York Peninsula, Upper Jardine R, 142° 36′E 11° 36′S, 26 Oct 1979, Molds MS, Molds BJ [MV]; 11 MS, 3 FS (eggs examined with SEM), 14 L, Cockatoo Ck, Telegraph Crs, 142° 27′E, 11° 39′S, 5, 6, 11 Feb 1992, Cartwright D, Wells A [MV]; 1 FI, 2 FS, 3 L, Bertie Ck at 250 m & 1 km SE Healthlands H.S., 142° 35′E, 11° 45′S, 4, 5, 11, 16 Feb 1992, Cartwright D, Wells A [MV]; 8 MS, 1 FS (reared, with L exuvium), 17 L (4 L: EPH-1094, ANVC), Bertie Ck, Telegraph Crs, 142° 30′E, 11° 50′S, 5, 12 Feb 1992, Cartwright D, Wells A[MV]; 2 MS, 1 FS, 5 L, McDonnell Ck & Cockatoo Ck Jct, 8, 13, 18 Feb 1992, Cartwright D, Wells A [MV]; 1 FI, 7 MS, 3 FS, 5 L, Dulhunty R, Telegraph Crs, 142° 30′E, 11° 50′S, 8, 9, 10, 15, 18, 19 Feb 1992, Cartwright D, Wells A [MV]; 3 MS, 2 FS, Hann R, 70 mi S of Coen, 23 Jun 1970, LeSouef [MV]; 1 L, Gonshot Ck, Telegraph Crs, 12 Feb 1992, Cartwright D, Wells A [MV]; 1 L, Cholmondeley Ck, Telegraph Crs, 8 Feb 1992, Cartwright D, Wells A [MV].
Redescription. Male imago: Body length 7.0 mm. General body colour light yellow with dark purplish brown markings. Dorsal compound eyes orange in alcohol, length 0.92 mm, width 0.78 mm, height 0.55 mm; distance between dorsal eyes 0.05 mm (B/D=0.06); basal compound eyes black, length 0.66 mm, height 0.31 mm. Thorax colour light yellow with various dark brown markings; pronotum posteromedially deeply concave, with submedian and lateral dark brown stripes; mesonotum marginally dark brown; pleura and sterna with various irregular dark brown markings and spots including mesopleural vertical markings and transverse sternal stripes; metanotum dark brown. Forewings hyaline, length 6.6 mm, width 2.2 mm; longitudinal veins hyaline (Sc and R1 basally stained light yellowish); crossveins dark purplish brown; crossveins C-Sc 12, strongly infuscated; crossveins Sc-R1 12, infuscated; crossveins R1-R2 10, infuscated; 3 crossveins in basal stigmatic area and near bullae somewhat concentrated and forming large dark purplish brown markings. Hindwings hyaline, length 1.3 mm, width 0.7 mm; veins hyaline; crossveins C-Sc 4-5, apically concentrated; crossveins Sc-R1 5; costal area round with indistinct costal projection at mid-length; Rs 0.31 mm; R1 0.44 mm; MPs 0.31 mm; MP1 0.47 mm. Legs light yellow; dorsal forefemora with purplish brown markings basally, at mid-length and apically. Abdomen colour white to light yellow with purplish brown markings; terga purplish brown with broad longitudinal median white stripe and anterosublateral and lateral white areas, with anterolateral dark purplish brown spots. Sterna with dark purplish brown longitudinal stripe (margins not clearly demarcated); sterna I posterior 1/2 dark purplish brown. Penes (Fig. 1C) Y-shaped, length 0.14 mm, apically pronounced, with pointed light yellow processes apicolaterally, with apical and dorsolateral light brown areas; forceps light yellow; forceps segment 1 0.63 mm, segment 2 0.06 mm, segment 3 0.06 mm; forceps segment 1 basally broad and apically slender with distinct constriction at 2/3 apically, arched dorsally at constriction. Caudal filaments light yellow with alternating broad and narrow dark brown markings.
Female imago: Body length 6.3-6.5 mm. General body colour and markings similar to male. Compound eyes width 0.27 mm; distance between compound eyes 0.83mm(B/D= 3.11). Forewings length 7.3 mm, width 2.5 mm; venation, vein colour and markings similar to male. Hindwings hyaline, length 1.3 mm, width 0.8 mm; veins hyaline; venation similar to male. Abdomen colour and markings similar to male. Caudal filaments with alternating broad and narrow dark brown markings.
Larva: Male body length 7.0-7.2 mm; caudal filaments 5.6 mm. Female body length 7.5-9.3 mm; caudal filaments 6.3- 6.9 mm. General body colour light yellow with dark purplish brown markings. Head light brown, length 1.27 mm, width 1.59 mm, with dark brown areas between lateral ocelli, at sublateral vertex and along ecdysal line. Male compound eyes width 0.48 mm; distance between compound eyes 0.52 mm (B/D=1.10). Female compound eyes width 0.24 mm; distance between compound eyes 1.11mm(B/D=4.67). Antennae length 4.0 mm; 2nd segment dark brown. Clypeus length 0.40 mm, basal width 0.68 mm, apical width 0.64 mm, with well developed sublateral projections, with ca. 8-10 hair-like setae subapicolaterally, with ca. 4-6 hair-like setae subbasolaterelly. Maxillary palp segment 1 0.26 mm, segment 2 0.29 mm, segment 3 0.12 mm. Labial palp segment 1 0.38 mm, segment 2 0.27 mm, segment 3 0.20 mm. Pronotum length 0.80 mm, width 1.59 mm, 1/2× longer than wide, with submedian and sublateral dark brown irregular markings; anterolateral margin round; lateral margin white with row of sparse hair-like setae. Mesonotum with submedian dark brown irregular markings and dots; forewingpads with small black spots basally and centrally. Thoracic sterna with various dark brown markings. Forefemora 1.32 mm, foretibiae 1.32 mm, foretarsi 0.56 mm, foreclaws 0.24 mm; forefemora with dark brown stripes dorsally and ventrally at basal, midlength and apical areas. Abdominal terga (Fig. 2C) with broad submedian dark brown stripes and with light areas along median line, lateral margins and anterosublateral areas (median light area somewhat Y-shaped containing paired small dark dots anterosubmedially); abdominal segment VI-IX with well developed posterolateral projections. Abdominal sterna with dark brown stripe along median line. Gills apicomedial expansion strongly developed (gill 4 expansion rate ca. 0.19); lateral tracheae dark brown and distinct; margins with dense fine setae only in apical area of expansion (Fig. 3C). Caudal filaments light yellow, with alternating broad and narrow dark brown markings.
Diagnosis. The male adult of
Distribution. QLD.
Remarks. Although we were unable to locate the holotype material of this species in the Stockholm Museum, the adult and larval material from Queensland could readily be identified based on the original description of Ulmer (1916). The suggested common name of this species is the ‘common hornless burrowing mayfly.’
Ulmerophlebia pipinna Suter (Figs. 1D, 2D, 3D)
20 (catalogue); Hubbard & Campbell 1996: 32 (catalogue).
Type material. Holotype: Male imago (wings and legs on slides, T-8953), Australia, Victoria, Grampian Mountains, Second Wannon River (GR. 547396), 25 Sep 1977, Suter PJ, Suter DN [MV]. Paratypes: 2 male imagos (T-8954), same data as holotype [MV]; 1 larva (T-8955), Australia, SE South Australia, Hitchcocks Drain, 22 Sep 1977, Suter PJ, Suter DN[MV].
Other material. VIC: 5 MI, 1 FI, 1 MS, Yea R at 7 km S of Glenburn, 1 Dec 1972, Neboiss A [MV]; 2 FI, Tarago R at 7 km W of Neerim, 1 Mar 1972, Neboiss A [MV]; 1 MI, Hopkins R at Hopkins Falls, 6 Mar 1978, Calder AA [MV]; 1 FI, Maramingo Ck at 5 km NE of Genoa, 29 Mar 1974, Neboiss A [MV]; 1 MI, Erinunderra R at 15 km N of Club Terrace, 11 Nov 1975, Blyth J [MV]; 1 FI, Yarra R below Upper Yarra Dam, 28 Feb 1976, Neboiss A [MV]; 5 L, Yarra R at Hazlewood Rd, 17 Feb 1978, Dean J [MV]; 1 MI (reared, with L exuvium), Yarra R at Hazlewood Rd, 23 Feb 1984 [MV]; 3 MI, Starvation Ck, 20 Feb 1976, Dean J [MV]; 1 MI (reared, with L exuvium), 1 FI (reared, with L exuvium), Starvation Ck, 19 Mar 1976, Dean J [MV]; 1 MI, Healesville, Coranderrk Reserve, 26 Mar 1976, Aankin [MV]; 4 FI, Delegate R at 8 km SW of Bendock, 15 Dec 1976, Neboiss A [MV]; 1 MI, 1 FI, Diamond Ck at 7 km SE of Gembrook, 31 Jan 1979, Wells A, Neboiss A [MV]; 1 FI, Gellibrand R at Asplin Ck Jct, 28 Jan 1982, Neboiss A [MV]; 2 MI (reared, with L exuvium), Watts R at Fernshaw, 4 Mar 1976, Dean J [MV]; 2 FI (reared, with L exuvium), Badger Ck d/s weir, 18 Jan 1980; 1 FI (reared, with L exuvium), Armstrong Ck, 16 Dec 1983, Dean J [MV]; 2 L (EPH-0957: ANVC), Mt Buffalo, Crystal Brook, 7 Jun 1977, Dean J [MV]; 1 MI, 1 MS, Tanjil R Jct, 10 km N of Willow Grove, 18 Dec 1973, Neboiss A [MV]; 2 L (EPH-0971: VVC), LaTrobe R (Survey St. 17), Tanjil R, 19 Feb 1973 [MV]; 1 MI (reared, with L exuvium), LaTrobe R at Br of SW Willow Grove, 10 Dec 2000, Bae YJ, Campbell I [MU]; 1 L, Grampians, Stony Ck, 26 Nov 1990, Cartwright D [MV]; 2 L, Wannon R at Serra Rd, 4 Dec 1983, Dean J, Cartwright D [MV]; 2 L, Snobs Ck Falls, 145°53′E, 37°18′S, 27 Dec 1982, Neboiss A [MV]; 2 L, Glenelg R, Big cord, 7 Mar 1995 [MV].
Redescription. Male imago: Body length 8.5-8.7 mm. General body colour light yellowish brown with dark purplish brown markings. Dorsal compound eyes orange in alcohol, length 0.98 mm, width 0.78 mm, height 0.42 mm, completely connected at posterior part (B/D=0); basal compound eyes black, length 0.52 mm, height 0.36 mm. Thorax colour light yellowish brown with various dark purplish brown markings; pronotum dark purplish brown, posteromedially deeply concave; mesonotum posterolaterally purplish brown; metanotum dark purplish brown; pleura light yellowish brown to purplish brown; sterna purplish brown. Forewings hyaline, length 8.7 mm, width 3.0 mm; veins stained light yellowish brown; crossveins C-Sc, Sc-R1 and R1-R2 dark purplish brown (basally darker in colour); crossveins C-Sc 18, basal 1/2 infuscated; crossveins Sc-R1 16, basal 1/2 slightly infuscated; crossveins R1-R2 13, slightly infuscated. Hindwings hyaline, length 1.9 mm, width 1.0 mm; veins hyaline; crossveins CSc 10, apical 7 crossveins concentrated, stained slightly yellowish brown; crossveins Sc-R1 5, stained slightly yellowish brown; costal area rounded with indistinct costal projection 1/3 basally; Rs 0.27 mm; R1 0.75 mm; MPs 0.28 mm; MP1 1.03 mm. Forelegs yellowish brown; dorsal forefemora with purplish brown markings at mid-length and apically; apical tibiae, tarsal segment 1 and 5, terminal tarsal segments 2, 3 and 4 and claws purplish brown. Forefemora 1.67 mm, foretibiae 2.83 mm, foretalsal segment 1 0.13 mm, segment 2 0.97 mm, segment 3 0.93 mm, segment 4 0.70 mm, segment 5 0.30 mm, foreclaws 0.13 mm. Abdomen colour light yellowish brown with dark purplish brown markings; terga purplish brown with broad longitudinal median light yellow stripe, with broad C-shaped dark purplish brown markings, with indistinct anterolateral dark purplish brown spots. Sterna with indistinct dark purplish brown longitudinal stripe (margins not clearly demarcated). Penes (Fig. 1D) V-shaped, slender, dorsoventrally flattened, length 0.16 mm, light purplish brown laterally; forceps light yellow; forceps segment 1 0.68 mm, segment 2 0.08 mm, segment 3 0.08 mm; forceps segment 1 broad basally and slender apically with distinct constriction at mid-length, arched dorsally at constriction; segments 2 and 3 not clearly demarcated. Cerci 9.5 mm, light purplish brown basally, light yellow apically, with alternating dark and light joints.
Female imago: Body length 8.6 mm. General body colour and markings similar to male. Compound eyes width 0.22 mm; distance between compound eyes 1.08mm(B/D=4.91). Forewings length 9.4 mm, width 3.2 mm; venation, vein colour and markings similar to male; Hindwings hyaline, length 1.8 mm, width 0.9 mm; veins hyaline; venation similar to male. Abdomen with distinct C-shaped markings similar to male. Caudal filaments light yellow with alternating dark and light joints.
Larva: Male body length 9.2-9.8 mm; caudal filaments 7.6- 10.0 mm. Female body length 9.5-11.2 mm; caudal filaments 9.2-13.2 mm. General body colour light yellow to light purplish brown with dark purplish brown markings. Head light purplish brown, length 1.40 mm, width 1.75 mm; clypeus dark purplish brown. Male compound eyes width 0.58 mm; distance between compound eyes 0.43 mm (B/D=0.74). Female compound eyes width 0.23 mm; distance between compound eyes 1.35 mm (B/D=6.00). Antennae length 4.0 mm; segments 1 and 2 light purplish brown. Clypeus length 0.45 mm, basal width 0.90 mm, apical width 0.83 mm, with well developed sublateral projections, with ca. 10 hair-like setae subapicolaterally, with ca. 15 hair-like setae subbasolaterelly. Pronotum length 0.83 mm, width 1.86 mm, with submedian and sublateral dark brown irregular markings; lateral margin white with row of sparse hair-like setae. Mesonotum with submedian dark brown irregular markings and dots. Forefemora 1.50 mm, foretibiae 1.38 mm, foretarsi 0.55 mm, foreclaws 0.25 mm. Abdominal terga (Fig. 2D) light yellow with broad median light area, with broad C-shaped dark purplish brown submedian markings, without submedian dark spots; abdominal segment VI-IX with moderately developed posterolateral projections. Abdominal sterna with dark purplish brown submedian stripes along median line containing submedian dark spots near anterior margin. Gills (Fig. 3D) apicomedial expansion not developed (gill 4 expansion rate 0), lateral tracheae invisible; margins with dense fine setae entirely on outer margin and on apical 1/2 of inner margin. Caudal filaments light yellow.
Diagnosis. The male adult of
Distribution. VIC, SA.
Remarks. The suggested common name of this species is the ‘southern hornless burrowing mayfly.’
Key to known species of Ulmerophlebia
Male imago
1. Male penes greatly reduced, < 1/5 × length from base to constriction of basal segment of forceps (Fig. 1B); QLD ???? ???????????????????????????????????????????????????????????????????
- Male penes Y-shaped or V-shaped, 1/3-1/2×length from base to constriction of basal segment of forceps ?????????? 2
2. Male penes V-shaped, without apicolateral processes or spines (Fig. 1D); VIC, SA ????????????????????????????
- Male penes Y-shaped, with apicolateral processes or spines ???????????????????????????????????????????????????????????????????????????????? 3
3. Male penes with apicolateral processes (Fig. 1C); QLD ??? ????????????????????????????????????????????????????????????????
- Male penes with apicolateral spines (Fig. 1A); NSW, VIC ????????????????????????????????????????????????????????????????
Larva
1. Gills apicomedial expansion not developed (gill 4 expansion rate 0), lateral tracheae invisible (Fig. 3D); abdominal terga with broad median light area, with broad C-shaped dark purplish brown submedian markings, without submedian dark spots (Fig. 2D); VIC, SA ..........
- Gills apicomedial expansion moderately to strongly developed, lateral tracheae visible or invisible (Fig. 3AC); abdominal terga colour pattern variable ???????????????? 2
2. Gills apicomedial expansion strongly developed (gill 4 expansion rate ca. 0.19), lateral tracheae distinctly visible (Fig. 3C); abdominal terga with broad median light area and anterosublateral light areas with submedian dark spots (Fig. 2C); QLD ?????????????????????????????????
- Gills apicomedial expansion moderately developed (gill 4 expansion rate ca. 0.08-0.10), lateral tracheae visible or invisible (Fig. 3A, B); abdominal terga colour pattern various ???????????????????????????????????????????????????????????????????? 3
3. Gills lateral tracheae invisible (Fig. 3A); abdominal terga with broad median light area and anterosublateral light areas with submedian dark spots (Fig. 2A); NSW, VIC ????????????????????????????????????????????????????????????????????
- Gills lateral tracheae ambiguously visible (Fig. 3B); abdominal terga with W-shaped broad median dark area (Fig. 2B); QLD ?????????????????????????????????????????????????????