Nitrogen is an essential element for living creatures. However, an excess of nitrogen from various sources can cause environmental problems such as eutrophication. Furthermore, when nitrogen compounds enter environmental water in the form of nitrate, they contaminate drinking water sources [1].
Nitrate is removed through various technologies including ion exchange, reverse osmosis, biological denitrification, and chemical reduction [2-5]. However, ion exchange and reverse osmosis require frequent regeneration of the media and generate secondary brine waste. Biological denitrification, the most widely used method, requires intensive maintenance and a constant supply of organic substrates. Moreover, the microbial processes are generally slower and sometimes less complete than chemical reduction. Of the various chemical reduction methods proposed for the effective removal of nitrate, zero-valent iron (ZVI) has attracted the most interest. The use of nanoscale zero-valent iron (NZVI) has several advantages over microscale ZVI, particularly in terms of its high level of stable reactivity [6].
However, the end-product of this reaction is sometimes unfavourable. We conducted a previous study on the final product of nitrate reduction, and ammonia, which is undesirable, was the main end product [7]. Therefore, it is required to consider further treatment for complete removal of the ammonia produced.
Recently, membrane contactors have been considered as an attractive alternative for the removal of volatile contaminants such as ammonia [8]. Membrane contactors allow direct contact and mass transfer between gaseous and liquid phases without dispersing one phase into the other [9].
Compared to conventional air stripping, ammonia stripping using membrane contactors provides a number of advantages. These include a larger interfacial area per unit volume, which provides fast removal of the volatile contaminant, and independent control of gas and liquid flow rates without causing any flooding, loading, or foaming. This also contributes to lower capital cost and ease of operation [10]. In particular, ammonium can be recovered in the form of ammonium salt in liquid/liquid membrane contactor systems. Due to the increase of global demand for nitrogenous fertilizer, from 10 Mt N in 1960 to 90 Mt N in 1998 [11], control over the point sources of N and P has shifted from removal to recovery, with a particular emphasis on improving the sustainability of agricultural activities.
In this study, polytetrafluoroethylene (PTFE) membranes were used in order to contact the aqueous solutions of ammonia and the receiving solution. Diluted solutions of sulfuric acid
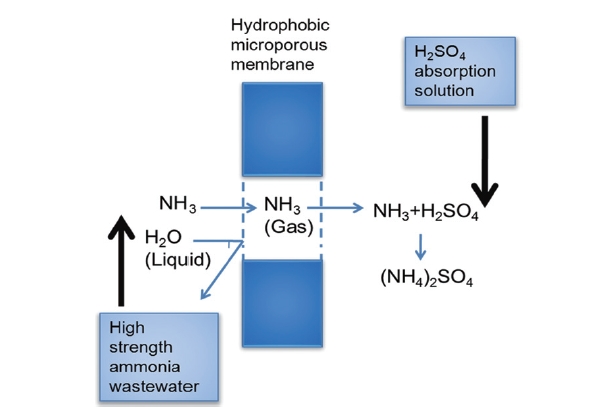
were used as the receiving solution. The principle of the removal of the ammonia from the feed to the receiving solution is illustrated in Fig. 1. The hydrophobic membrane separates two circulating phases: the feed that is aqueous phase containing ammonia on the shell side and the receiving solution, and a diluted solution of sulfuric acid on the lumenside. An air gap fills the pores of the hydrophobic membrane, which is not wetted by the aqueous solutions.
First, ammonia (NH3) diffuses from the bulk of the feed to the feed-membrane interface. NH3 volatilizes through the feedmembrane interface, diffuses across the air-filled pores of the membrane, and finally reacts immediately with sulfuric acid on the interface to form the non-volatile compound ammonium sulphate ((NH4)2SO4). Thus, the concentration of ammonia in the receiving solution is essentially zero. Total ammonia removal could be theoretically possible under this configuration, since the driving force for this liquid-gas-liquid membrane contactor operation is the difference in ammonia partial pressure between the feed and the receiving solution.
In this study, a tubular type membrane configuration was applied for ammonia stripping, and the feasibility of this membrane configuration was studied. The ammonia removal efficiencies under various operation conditions were evaluated. The pH, initial ammonia concentration, flow rate, and temperature difference were selected as reaction variables, and the crucial factors for operation were evaluated.
2.1. Synthesis and Characterization of NZVI
In this research, the mild chemical reduction of metal salts in a solution phase, which had chemical homogeneity as a key advantage, was used to prepare NZVI [12]. The NZVI was synthesized in a 500 mL flask reactor with four open necks. One of the necks was housed with a tunable mechanical stirrer at 60 to 500 rpm. For the reduction of ferric ions to NZVI, a dropping funnel was used (at a rate of 1 drop/sec) to introduce 250 mL of borohydride solution (358.4 mM) into 250 mL of ferric ion (Fe3+) solution (71.7 mM). The reduction reaction is expressed as follows [5]:
4Fe3+ + 3BH4- + 9H2O → 4Fe0 + 3H2BO3- + 12H+ + 6H2
The NZVI was collected by centrifugation of the solution after
20 min of aging. The collected particles were then washed three times with a large amount of degassed deionized water. The prepared NZVI was immediately used for a batch experiment. The NZVI was characterized with the help of a transmission electron microscope (TEM, Tecnai F20; Philips Electronics Co., Eindhoven, Netherlands), a particle size analyzer (ELS-8000; Otsuka Electronics Co., Osaka, Japan) and a Brunauer Emmett Teller (BET) surface area analyzer (Sorptomatic 1990; Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.2. Nitrate Reduction by NZVI
Batch tests for nitrate reduction were conducted in 1 L Schlenk flasks. The reactor was filled with 700 mL of nitrate solution, and the solution was purged with argon gas before the injection of NZVI. Then, 100 mL of an NZVI slurry containing 1 g of NZVI was added to the reactor, which contained 100 mg/L of nitrate. The solution was continuously stirred with a mechanical stirrer. Samples were taken periodically, filtered with 0.45 μm syringe filters, and then analyzed immediately. Argon gas was blown continually into the reactor to maintain an anoxic condition during the reaction.
Nitrate and nitrite concentrations were analyzed by means of ion chromatography (DX-120; Dionex Co., Sunnyvale, CA, USA). Ammonium and total nitrogen were analyzed using a standard method (ASTM D1426-03) and a spectrophotometer (DR-2010; HACH, Loveland, CO, USA) in accordance with the guidelines of the 20th edition standard method.
2.3. Membrane Contactor Configuration
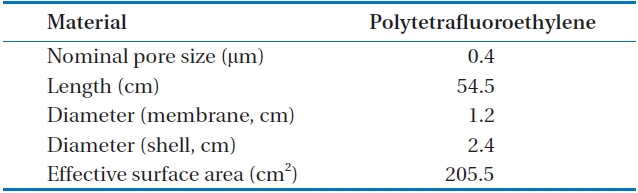
The tubular membrane used was made with PTFE and pore diameters of 0.4 μm, and 205.5 cm2 of effective surface area. The speciation of the membrane module is presented in Table 1.
[Table 1.] The speciation of membrane contactor module
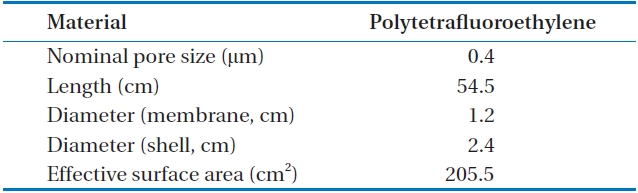
The speciation of membrane contactor module
Fig. 2 shows the membrane contactor system used in this study. The membrane module consists of PTFE tubular membrane encased in a polypropylene (PP) pressure vessel. The substrates were fed to the inside of membrane and the strip solution was supplied on the shell-side. Sulfuric acid (10%, w/w) was used as a stripping solution and flowed in the opposite direction of feed solution.
2.4. Operation Conditions of Membrane Contactor
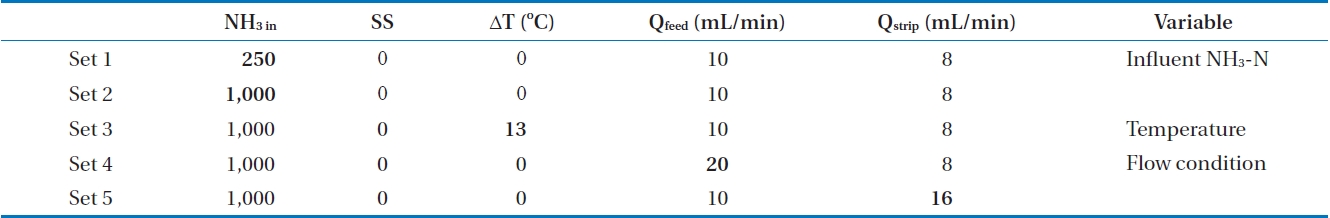
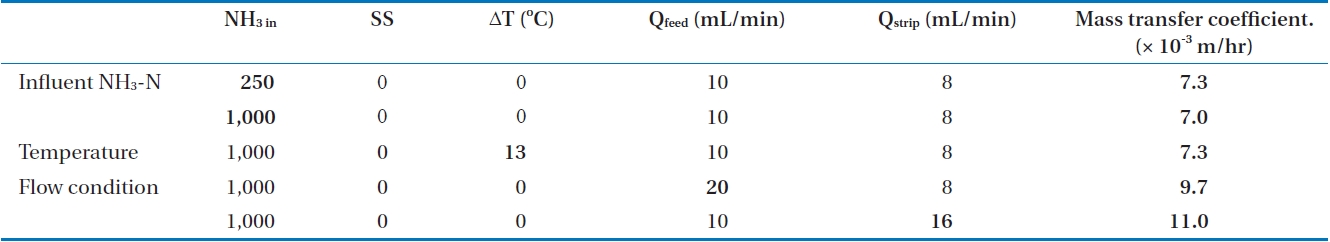
The feasibility of a membrane contactor to remove produced ammonia was evaluated under different operating conditions. Influent pH was selected to figure out the effect of free ammonia fraction on ammonia removal. The effect of initial ammonia concentration (250 and 1,000 mg/L), flow rate of feed solution (10 and 20 mL/min) and strip solution (8 and 16 mL/min), and temperature difference between feed and strip solution (0℃, 13℃) were also evaluated. The specific experimental conditions are shown in Table 2.
The change of ammonia concentration with time was used to calculate the overall mass transfer coefficient (km) as follows [13]:
in which C0 is the initial total ammonia concentration,
[Table 2.] The experimental conditions
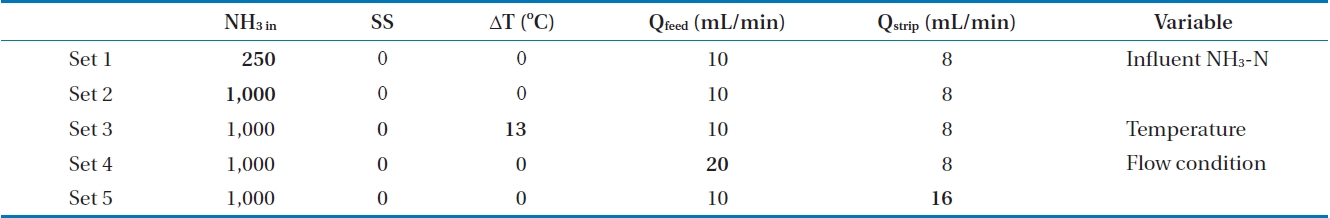
The experimental conditions
ume, the membrane length, and the feed velocity in the module, respectively. Eq. (1) was derived from mass balances of the module and the ammonia solution, assuming that the acid reservoir is big enough to neutralize all the ammonia.
3.1. Properties of NZVI and Its Nitrate Reduction Capability
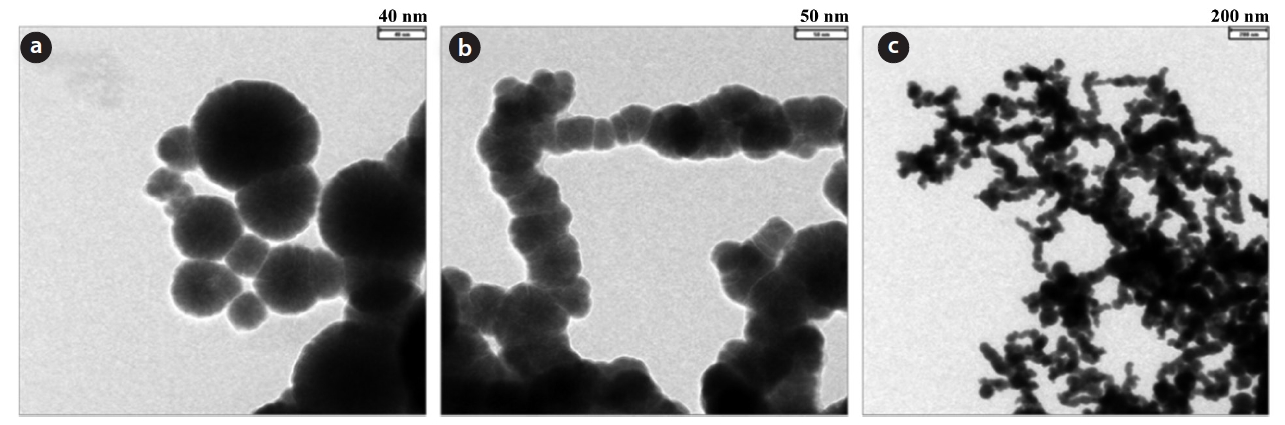
The TEM morphologies of the prepared NZVI in this study are shown in Fig. 3 with different magnifications. Laser scattering particle size analysis shows that the average diameter of the prepared NZVI was 16.7 nm. The BET surface area was 79.06 m2/g.
As shown in Fig. 3(c), NZVI is spherical with a chain network structure. The spherical shape and chain net morphology are similar to those observed in other studies [14]. The aggregation of NZVI in aqueous solution without a surfactant is a common behavior, but the accessibility of reactants to NZVI is good because the total surface area is unaffected due to the high porosity of the aggregates [15, 16]. The high reaction rate expected was confirmed by the high nitrate reduction rate of the subsequent experiments.
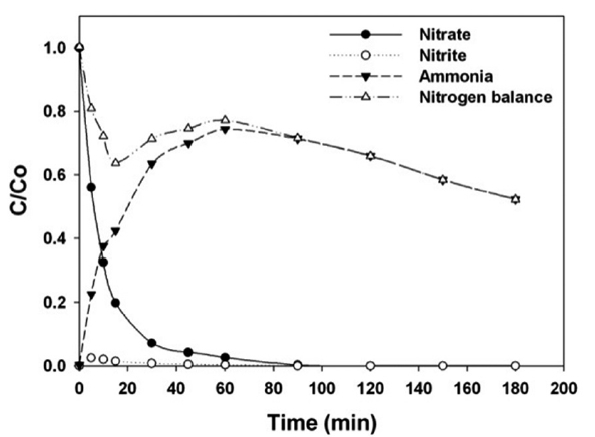
Fig. 4 shows the profile of aqueous nitrogen species in relation to the reaction time during nitrate reduction by NZVI. The reference conditions were a temperature of 30℃, an initial pH of 7, and an unbuffered status. More than 97% of the nitrate was removed within 1 hr and complete removal was obtained within 1.5 hr. Ammonia was produced as the main by-product, and its concentration decreased after 1 hr of reaction. Therefore, the suitable reaction time for the highest ammonia recovery is 1 hr.
3.2. Ammonia Stripping under Various pH and Its Related Kinetics
3.2.1. Effects of pH on ammonia removal by membrane contactor
For efficient removal of ammonia, it should be in its volatile form. Increasing pH or temperature to a point where all ammonium- nitrogen (NH4+-N) is in its volatile ammonia form (NH3) ensures this. The expression for free ammonia (FA) fraction of the total reduced ammonia is derived from known equilibrium expressions and is presented in Eq. (2) [17].
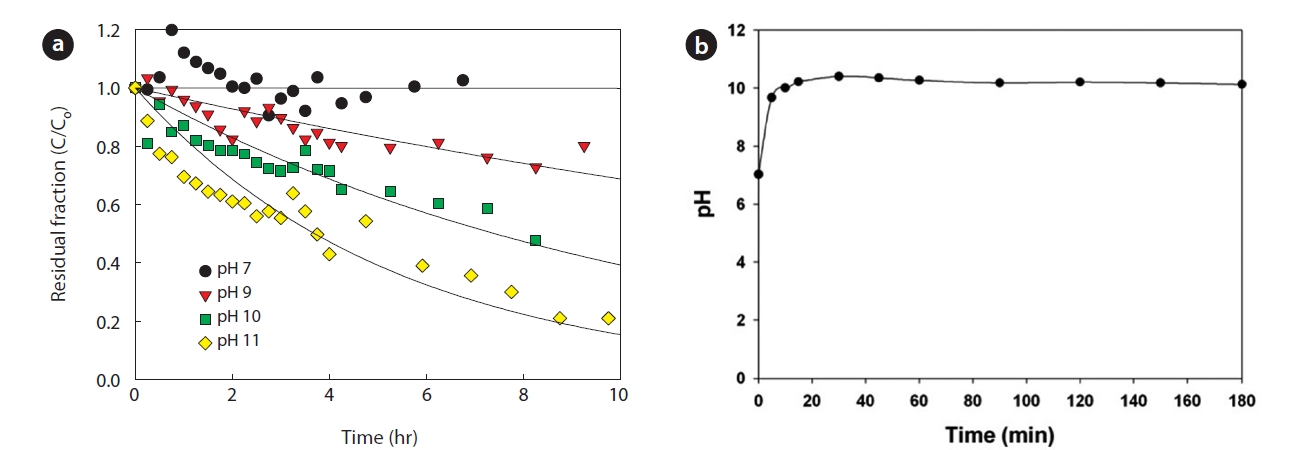
At constant temperature, pH is the one factor that influences FA fraction. Therefore, pH is one of the most important factors determining overall reaction efficiency. According to Eq. (2), high ammonia removal rate is expected at high pH. The ammonia removal rate is shown in Fig. 5(a). The ammonia removal rate was increased with the increased free ammonia fraction in the feed solution in the pH range of 7 to 11. Therefore, it was concluded that the high pH of the feed solution was essential for effective
ammonia stripping. All of following batch experiments were performed at pH 11 based on this preliminary test.
As shown in Fig. 5(b), the pH was increased to 10.5 during the nitrate reduction. The free ammonia fraction was 54.2% under pH 10. Therefore, it was possible to remove the produced ammonia effectively by the membrane contactor without pH adjustment.
3.2.2. Effects of influent concentration on mass transfer coefficient
Biological processes can be applied to remove ammonia and other contaminants from water and wastewater. However, the high concentration of ammonia has inhibitory effects on biological wastewater treatment processes. By using the membrane contactor, which is one of the conventional physico-chemical processes, selective dissolved pollutants can be removed. The ammonia removal kinetic described in Section 2.3 is not a function of concentration, but of residual fraction. To verify the effect of influent concentration on membrane contactor operation, the ammonia removal rate was observed at different initial ammonia concentrations of 250 and 1,000 mg/L.
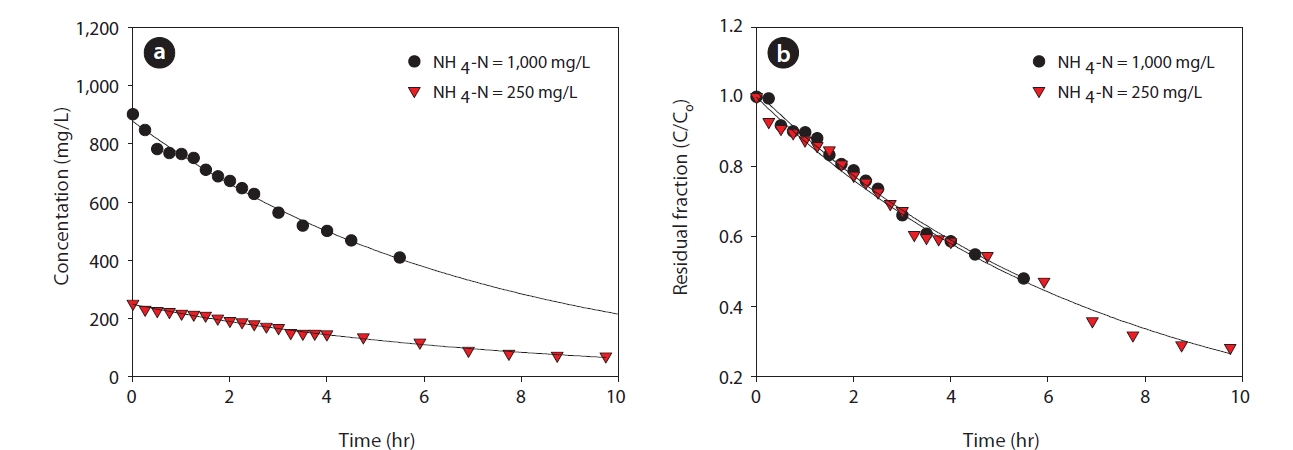
The amount of removed ammonia was increased with increased influent ammonia concentration in Fig. 6(a). However, the trend of residual fraction does not have a significant difference with different concentration. The mass transfer coefficient (km) decreased from 7.25 × 10-3 m/hr to 7.0 × 10-3 m/hr (3.4%) as the ammonia concentration increased from 250 to 1,000 mg/L. These results indicate the ammonia removal is mainly controlled by the ratio of ammonia concentration in the feed and strip solutions. Therefore, the membrane contactor is a suitable process for high strength wastewater.
3.3. Enhancement of Ammonia Stripping Efficiency by Changing of Temperature & Hydraulic Conditions
3.3.1. Prevention of osmotic distillation
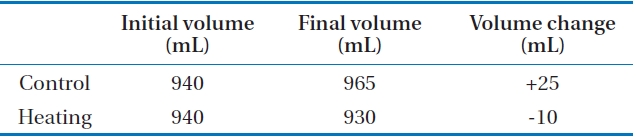
Osmotic distillation (OD) is an evaporative membrane contractor process, which is a coupled process in membrane absorption in treating high-strength ammonia-containing wastewater [18]. Due to the difference of vapour pressure between feed stream and strip solution stream, water molecules leak from the feed solution into the strip solution through membrane pores. (Fig. 7)
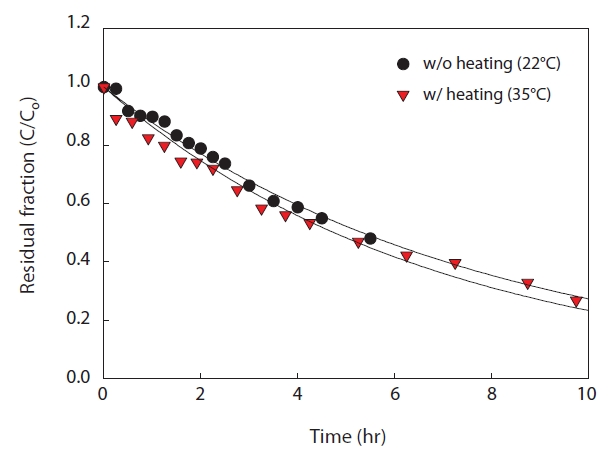
It was clear that the coupled OD brought negative effects to ammonia removal in the membrane contactor system. The removed ammonia could not be concentrated in the strip solution effectively due to the undesired transfer of water molecules by the osmotic distillation phenomenon. Therefore, the OD effects should be minimized to ensure successful operation of the membrane contactor process. Pre-heating of feed solution is one of the possible solutions to minimize the OD effect. Due to the high vapor pressure of the heated strip solution, water could not pass through the membrane from the feed solution. To verify this theory, strip solution was heated to 35℃, which was selected as the model temperature in mesophilic conditions (feed solution,
[Table 3.] The effect of osmotic distillation in membrane contactor operation
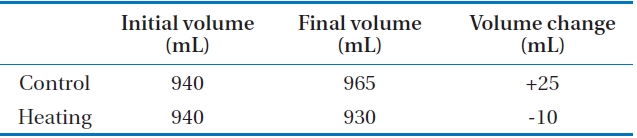
The effect of osmotic distillation in membrane contactor operation
22℃). Because of the temperature difference, the ammonia removal rate increased to 7.3 × 10-3 m/hr (4.4%). This result indicated that the effect of osmotic distillation on ammonia removal was not significant. (Table 3)
In case of the control experiment, the volume of feed solution increased by osmotic distillation phenomena. On the other hand, the volume of feed solution decreased in case of heating. Therefore, the osmotic distillation phenomenon was suppressed by temperature increase, even though it was not significant.
3.3.2. Increase of flow rate
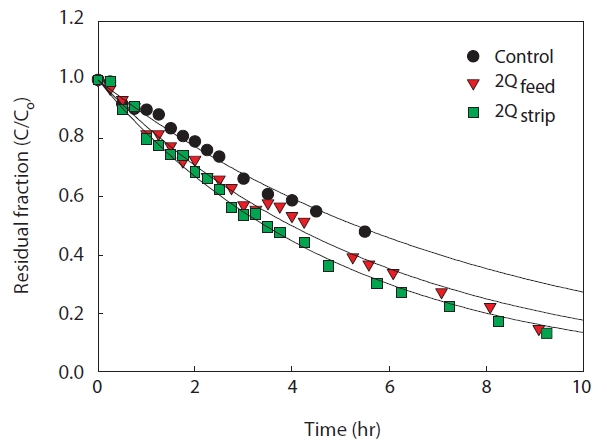
A large surface area of the membrane module can guarantee successful removal of ammonia in the membrane contactor system since the ammonia removal mechanism is evaporation through the membrane pores. Operation with high flow rate is one of the alternative ways to increase the contact between the strip solution and the wastewater stream. The flow rate was increased to twice that in control experiments for both of feed solution and strip solution.
As the flow rate increased for both solutions, the ammonia removal efficiency increased slightly, as shown in Fig. 8. Particularly, increase of strip solution flow rate showed great enhance-
[Table 4.] Summary of mass transfer coefficient under various operating conditions
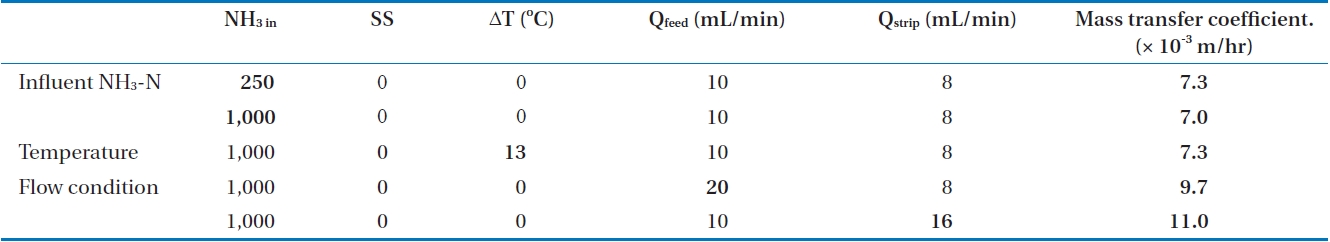
Summary of mass transfer coefficient under various operating conditions
ment of ammonia removal rate. This might be due to the strip solution being saturated at low flow rate. Therefore, the supply of fresh strip solution and high membrane surface area were essential for efficient membrane contactor operation.
The mass transfer coefficients under various operation conditions are summarized in Table 4. The mass transfer coefficient increased with the low influent ammonia concentration, suspended solid-free condition, suppression of osmotic distillation by temperature difference, and high flow rate for both strip and feed streams. Among these various operation conditions, higher flow rate led to greatest enhancement of mass transfer. This means that the most important factor for efficient ammonia removal in membrane contactors is the contact time, which could also be attained by the use of membranes with large surface areas. Because a tubular membrane module was applied in this study, it is hard to increase the membrane surface area. Alternatively, operation at high flow rate could be considered for effective ammonia removal.
In this research, complete removal and recovery of nitrate was studied by a combination of NZVI and a membrane contactor system. Ammonia was produced as the main end product of nitrate reduction by NZVI, and the produced ammonia was effectively removed in the tubular PTFE membrane contactor system. The removal efficiency of ammonia removal was mainly affected by feed solution pH. The solution pH of nitrate reduction by NZVI was maintained over 10, which is suitable for ammonia recovery by the membrane contactor. The effect of various operating conditions including influent ammonia concentration, temperature, and flow rate were investigated for the enhancement of mass transfer in the membrane contactor system. Among the various operation conditions, flow rate showed the most critical impact on ammonia removal by increasing the mass transfer coefficient over 50%. The ammonia removal rate can be maximized by optimizing operation conditions and changing membrane configuration. Therefore, the combination of NZVI and the membrane contactor system could be a solution for nitrate removal and recovery of valuable products.