It is believed that several human diseases come from polluted water, but water purification is a costly process. Many lakes in South Korea become a good environment for algal bloom in the summer due to the increasing eutrophication by hot weather. Eutrophication causes the growth of algae with increased turbidity of water, which provides a visually negative effect. Also, algae cause a clogging at the filtration plant or a bad taste in water [1].
When algae die off, they are decomposed by bacteria accompanying excess consumption of oxygen in water. These cause the oxygen shortage in water and cause a deposition of iron or manganese at the bottom of the lake. In addition, it even causes a mass death of fish from hypoxia [2]. Algae bloom is dominant throughout the hot summer at Nakdong River or Daecheong Lake in South Korea by eutrophication [3-7].
A mass growth of cyanobacteria at Gyongan stream in Paldang Lake created a red tide and destroyed the ecosystem on the fresh water. Also, many problems occurred in social, economical and environmental aspects. Among the problem by eutrophication, the recent big issue garnering great attention is the toxins by cyanobacteria [8-10]. Since it was first reported as animal damage by cyanobacteria in 1878, the damaging toxin has been continuously reported in livestock or wildlife around the world. Especially, drinking water from lakes or rivers containing cyanobacteria frequently happened in recent years.
Due to the controversy of the impact on the human body, cyanobacteria needs to be decreased efficiently or the eutrophication coming from the lake needs to be blocked. The conventional method for algae removal in lakes is usually the sedimentation method, which consists of spraying soil to coagulate down or using air bubble to float up or directly destroy the algae by a pesticide, toxin, organic, zeolite, or heavy metal [11-16].
Due to technical or economical reasons in Korea, the sedimentation method was widely used. But the algae in a basin consist of organics, which lack self-cleaning ability. They rot in the anaerobic environment with increasing biochemical oxygen demand (BOD) or chemical oxygen demand (COD). These cause secondary pollution to destroy the water ecosystem. For the improvement of water quality in the long term with protecting the ecosystem economically, algae removal research is extremely necessary.
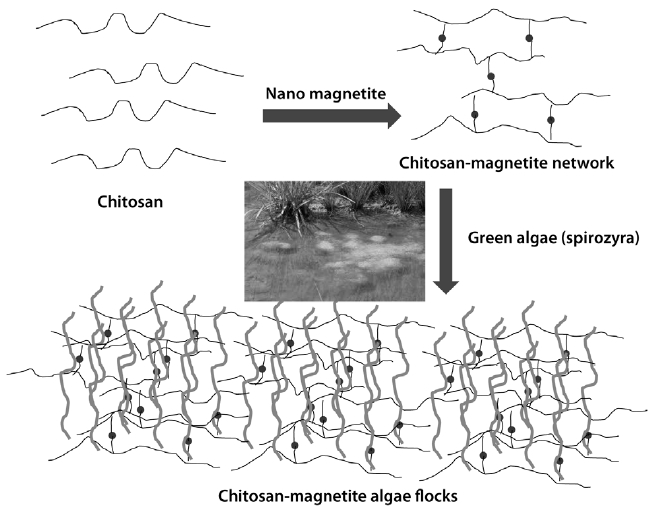
To overcome the current problem, we established a magnetic method and optimized the additive conditions. The main idea is to catch the large amount of algae by using the chitosan-magnetite network (Fig. 1). Chitosan tends to form a network structure when it combines with metal ion, and the chitosan have an amine group with the pKa value of 6.2. Thus, it tends to combine with the hydroxyl group in algae when the pH is greater than 6.2. However, the binding effect decreases when the pH becomes more than 7.5, because it competes with the algae proton detachment.
We represent the attachment relationship between the algae and the magnetite-chitosan complex by adjusting pH value, the resulting magnetite-chitosan-algae complexes were quickly taken out by magnetic bars. Using a large sized magnetite powder resulted in algae flocks being sunk down due to the heavy weight of the large magnetite powder. Therefore, we synthesized the small sized magnetite to enable the algae flock to float up and be caught more easily. This method can be applied to most algae in water, but it cannot apply to the rock or gravel attached algae. The major advantage is the high speed algae removal by
[Table 1.] Cultivation of algae
Cultivation of algae
extracting the water using the magnetic bar or industrial magnetic crane, and this algae flock could be useful for the bio fuel resources in the chemical industry.
FeSO4 · 7H2O, KNO3, and NaOH were analytical grade from Samchun Chemicals Co. (Seoul, Korea). Magnetite powder in this experiment were prepared by dissolving 0.5 M of FeSO4 · 7H2O in a 2 L beaker. This was mixed with 75 g of KNO3 and 350 g of NaOH. After mixing these, it heated up 90℃ on a hot plate to complete the reaction. The cation stock solution was prepared by dissolving chitosan, which is from Kumho Chemical Inc. (Daejeon, Korea). This chitosan de-acetylated amount is 80% with average molecular weight of 120,000 Da. Chitosan 1 g was dissolved in 1/20 dilute acetic acid to make 1 L of 1,000 mg/L cation solution.
2.1.1. Culture of algae solution
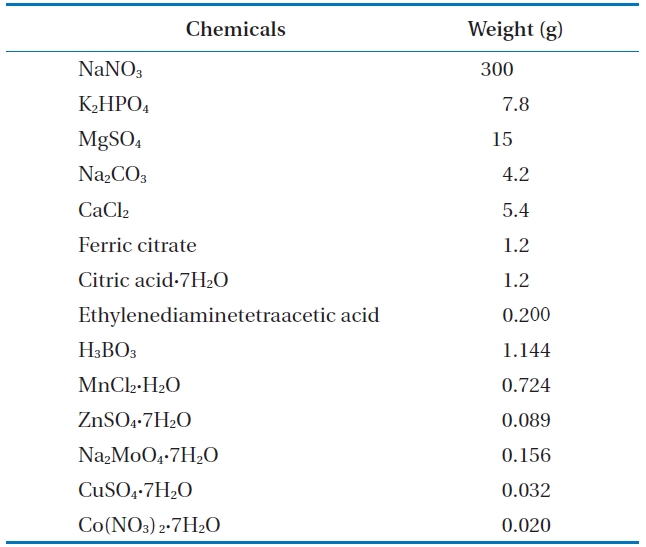
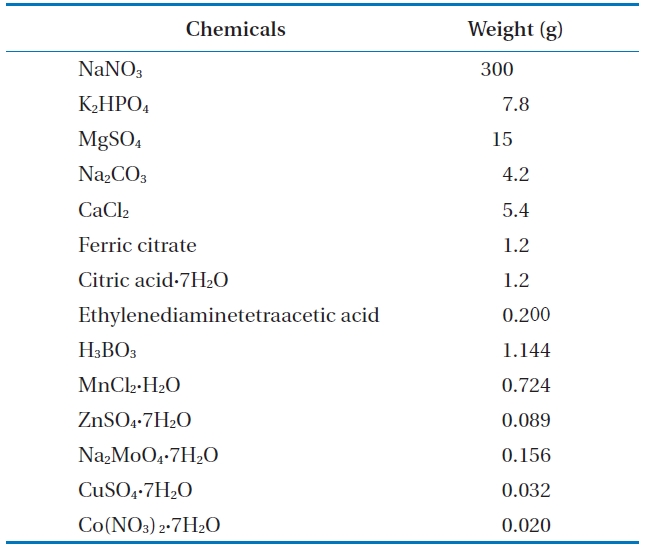
Since the green algae in this experiment show dormancy at the low temperature, we use Allen’s media method as in Table 1. Water is from a pond at Korea Research Institute of Chemical Technology (KRICT). Several ingredients such as in Table 1 were added and dissolved clearly. Pond water, which contains algae culture media, was transferred to a 15 L drinking water bottle as shown on Fig. 2. We added microcystis such as cyanobacteria and then mixed it evenly. This is grown in a greenhouse at a temperature of 25?28℃ for 15 days with exposure to sunlight for photosynthesis.
The green algae in the water need to be extracted by the magnetic method. First, we dispensed the algae solution into a 1 L mass cylinder and a magnetite was inserted into the large flock. To find out the optimum condition of algae out of the water, we modified the pH, cation concentration, and magnetic powder concentration. Several kinds of analysis were performed, which were pH, turbidity, chlorophyll-a, plots, total nitrogen, total phosphorus, and COD. The pH meter was a Thermo ORION 3-Star meter (Thermo Scientific, Waltham, MA, USA) and the turbidity meter was LaMotte 2020e (Chestertown, MA, USA). Floats, chlorophyll-a, total nitrogen, total phosphorus, and COD were measured by standard methods at EPA 815-R-05-004 January 2005 “Manual for the certification of laboratories analyzing drinking water.”
2.2.1. pH variation experiment
One liter of the algae water was dispensed to a mass cylinder. 0.25 g of magnetic powder and 10 mL of cation solution were mixed to prepare 0.025% of magnetite in a solution with a concentration of 10 mg/L. Then we adjusted pH using diluted sodium hydroxide and sulfamic acid to 6.0, 7.0, 8.0, 9.0, 10, and 11 in each sample and then mixed to coagulate. After that, the neodymium magnetic bar was inserted into a mass cylinder, we moved the magnetic bar up and down to remove the algae from the water and the performance was shown by comparing the original solution.
2.2.2. Cation concentration
One liter of algae water was dispensed into a mass cylinder. 0.25 g of magnetic powder and the cation concentration for each 2.5, 5.0, 10, 15, 20 mg/L solutions were mixed to prepare 0.025% of the magnetite concentration. We adjusted pH to 7.0 using diluted sodium hydroxide and sulfamic acid and we quickly mixed these. Afterwards, a neodymium magnetic bar was inserted into a mass cylinder. This separate algae flock from water and the performance was shown by comparing the original solution.
2.2.3. Magnetic powder concentration
The magnetite concentrations were varied by 0.01%, 0.025%, 0.05%, 0.075%, and 0.10%, while the cation concentration was fixed at 10 mg/L. The pH was adjusted to 7.0 using diluted sodium hydroxide and sulfamic acid. Next, a neodymium magnetic bar was inserted into a mass cylinder. The performance was shown by comparing it with the original solution.
3.1. Characteristic of Magnetic Powder
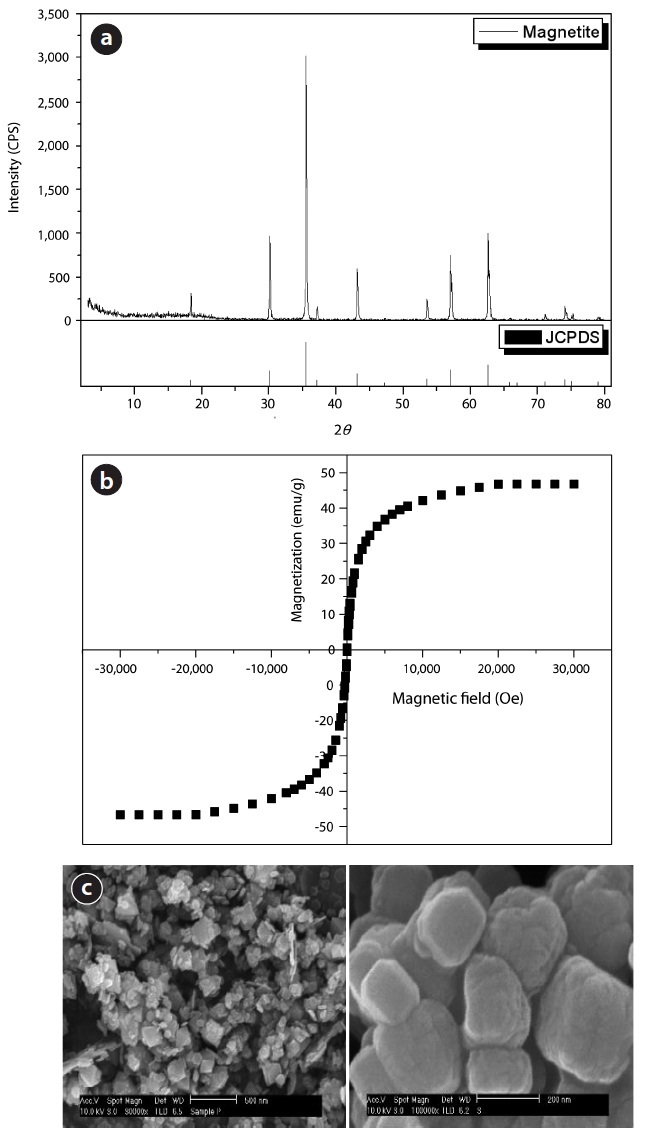
X-ray powder diffraction was performed by Rigaku D/MAX- 2200V (Tokyo, Japan) X-ray diffraction-meter to structure determination. The 2
Fig. 3(b) is the superconducting quantum interference device (SQUID, MPMS-7; Quantum Design, San Diego, CA, USA) for the magnetic property measurement. We measured magnetic properties in conditions between +30 kOe and -30 kOe under a magnetic field. The magnetic hysteresis curve showed the soft ferromagnetic for the synthesized magnetite nanoparticles. This showed specific magnetic value when the magnetic field occurred. Otherwise, the magnetic value became zero when the magnetic field was removed. We obtained the magnetic value 46.7 emu/g at the magnetic field of 30 kOe. We believe that this magnetite could be mixed with the algae floc in order to separate algae flock from water rapidly by the magnetic method.
Synthesized magnetite morphology was observed by scanning electron microscope (SEM; Philips XL30S FEG, Eindhoven, Netherlands), as shown on Fig. 3(c, d). The morphologies of the synthesized magnetite were oval, rectangle, and hexagonal mixtures because a dispersion agent was not added to the thermal synthetic procedure [17, 18].
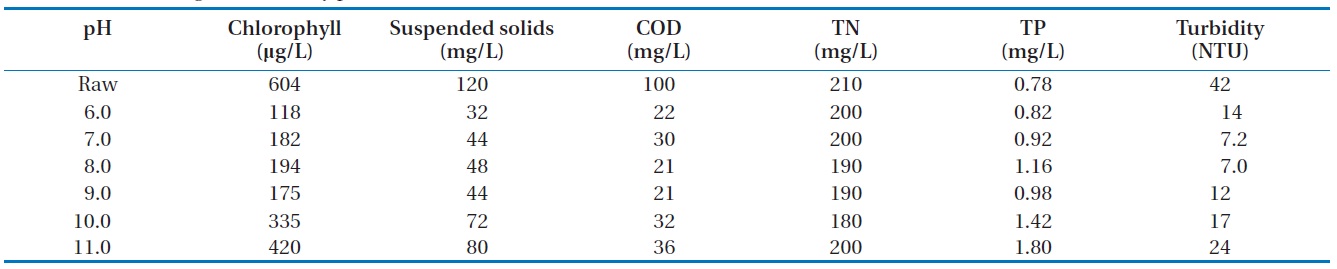
3.2.1. pH variation
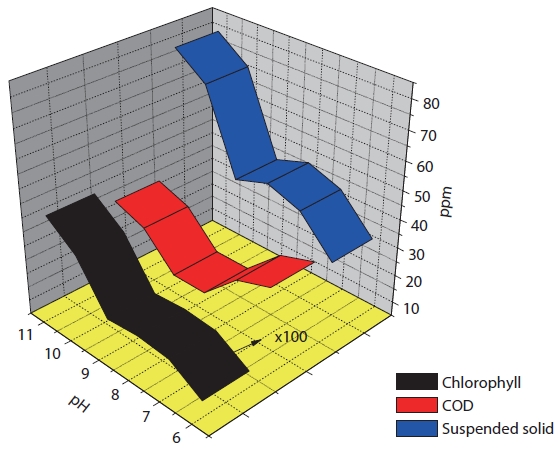
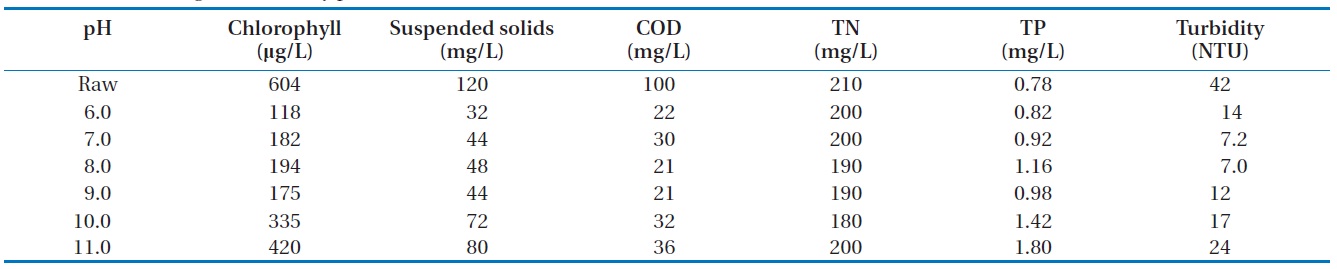
We fixed the amount of magnetic powder or cation and varied the pH to 6?11, as shown in Table 2 and Fig. 4. This showed that the algae removal rates were different with pH variation. The highest removal rate was demonstrated in the range of pH 6.5? 7.5 and the COD or turbidity was in accordance with the removal rate. But the total nitrogen (TN) or total phosphorous (TP) was not affected by the pH variation. For the removal of negatively charged algae, the neutral pH was the best for the flock formation. So it could be thought that the best algae removal efficiency
would come from the neutral pH. The low COD indicated that the organics in algae were successively removed.
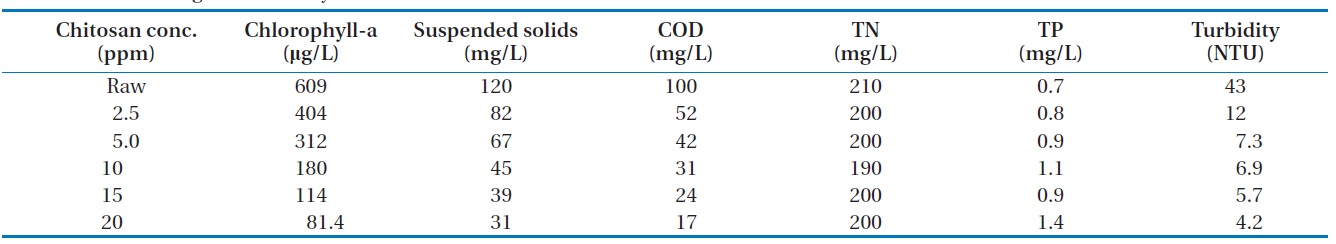
3.2.2. Cation concentration variation
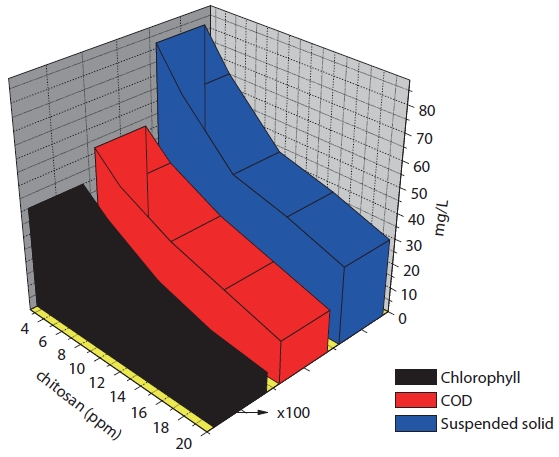
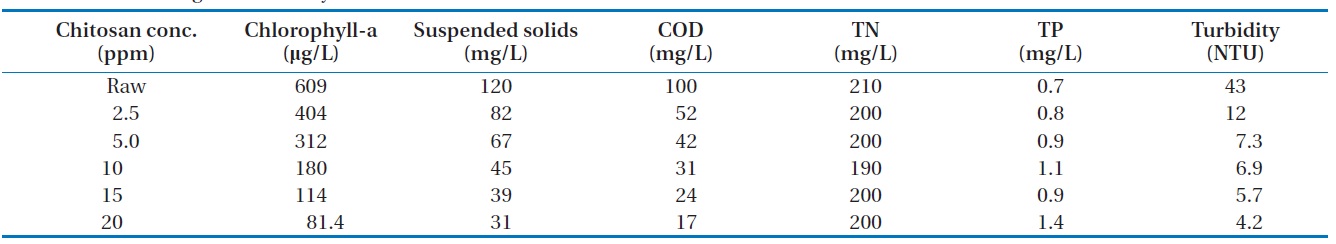
Table 3 and Fig. 5 showed the results of algae removal by variation of cation concentration. The magnetic powder concentration was fixed and the solution pH = 7. The algae suspension, COD, and turbidity were decreased when the cation concentration increased. However, the total amount of nitrogen or phosphorus was not affected. So, it was confirmed that the positive charged chitosan solution could be used for the algae removing agent as an eco-friend material. The results indicated that the removing rate did not increase at the chitosan concentration of over 10 mg/L. Therefore, with regards to economical use, using the chitosan concentration of 10 mg/L was the best.
[Table 2.] Results of algae removal by pH variation
Results of algae removal by pH variation
[Table 3.] Results of algae removal by variation of chitosan concentration
Results of algae removal by variation of chitosan concentration
[Table 4.] Results of algae removal by variation of magnetic powder concentration
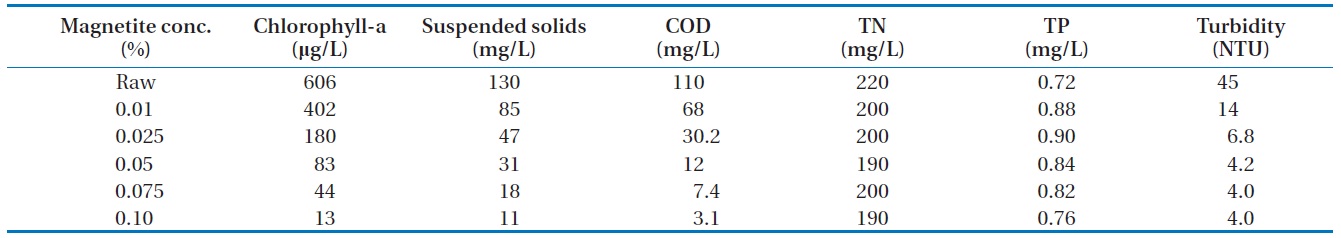
Results of algae removal by variation of magnetic powder concentration
[Table 5.] Magnetic algae removal time by variation of magnetite concentration

Magnetic algae removal time by variation of magnetite concentration
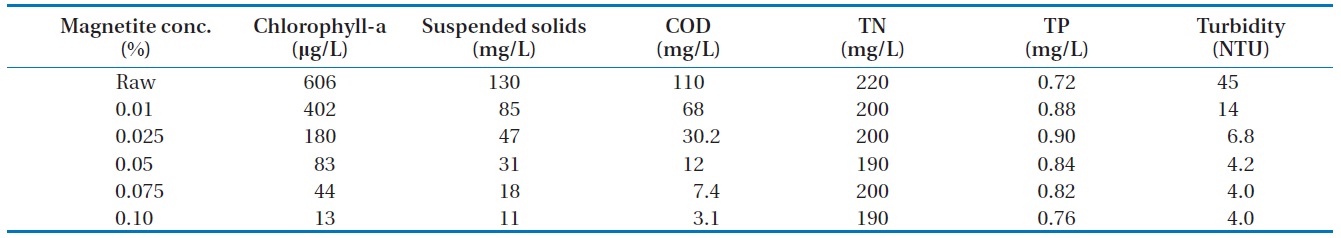
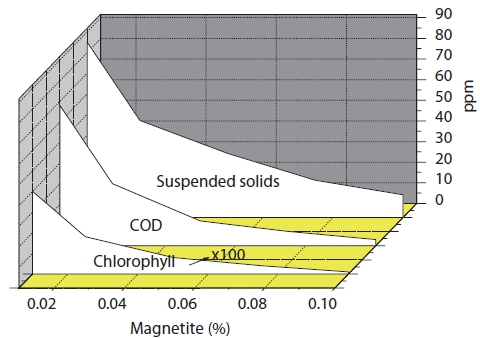
3.2.3. Magnetic powder variation
The chitosan concentration and pH were fixed. The magnetic powder concentrations were varied. The removal efficiencies of algae were shown in Fig. 6. The removal rate of algae and COD were affected when the magnetite concentration increased. The turbidity decreased when the magnetite concentration increased. The turbidity was continued at about 4 NTU when the magnetite concentration was greater than 0.05%.
It would be possible to lose the magnetite in the water during the algae removal treatment. This might create some drawback to contaminate water with metals. The magnetite concentration had to be less than 0.05% to meet the economic and environmental concern.
3.2.4. Rapid separation of algae
The neodymium magnetic bar was inserted to a 1 L mass cylinder, which contains algae solution. The magnetic bar was catching the algae flock from water and we measured the algae removal time by a stopwatch. The conventional sedimentation method took more than 2 hr to coagulate algae to sink down, but the magnetic separation method took only 30 sec as shown in Table 5. The magnetic separation method was the quickest among the current algae removal technology.
Various algae removal results were obtained by variation pH, cation concentration, and magnetic powder concentration. For removing algae from water rapidly with regard to both an economic and environmental way, we obtained the following optimized results. 1) To separate the algae, we synthesized magnetite by the hydrothermal method. The morphologies of magnetite are oval, rectangle, and hexagonal mixtures. The magnetize value was 78.2 emu/g when under a measurement condition of the 16.5 kOe magnetic field. It was confirmed that the algae was rapidly removed from water by the magnetite, which was automatically inserted in the algae flock. 2) The optimum pH was 6.5?7.5 for the algae to coagulate with the magnetite powder. The coagulated algae were removed by permanent magnets. 3) The removal rate increased when the chitosan concentration increased. But a chitosan concentration greater than 10 mg/L did not affect the superior rate. So, the economical amount would be less than 10 mg/L for the algae removal treatment. 4) The removal rate increased as the magnetite contents increased, but the turbid-ity did not change when using a magnetite amount greater than 0.05%. Therefore, the magnetite amounts needed to use less than 0.05% as we considered the environmental or economic effect. 5) The magnetic separation method was the quickest among the current algae removal technologies.