Osteosarcoma is the most common malignant bone cancer in children and adolescents (Thompson et al., 2002; Hauben et al., 2003). Over 80% of patients with osteosarcoma have metastatic or micrometastatic disease at diagnosis (Mehta et al., 1986; Kaste et al., 1999). For patients presenting with metastatic diseases or tumor recurrence, outcomes are far worse, with survival rates below 30% (Zhang et al., 2008). Current treatment for osteosarcoma includes chemotherapy and surgery. Unfortunately, major problems associated with chemotherapy persist, in particular, the cytotoxic effects of chemotherapy on normal tissues and organs. Thus, research has focused on the discovery of new agents and strategies to prevent the progression of osteosarcoma.
Our knowledge of the mechanistic control of invasion and metastasis in osteosarcoma is limited. Tumor growth, inva-sion, and metastasis require tumor cell proliferation, proteo-lytic digestion of the extracellular matrix (ECM), cell migra-tion through basement membranes into the circulatory system, and extravasation and growth at the metastatic sites (Liotta and Kohn, 2001). Matrix metalloproteinases (MMPs) have been known to contribute to this metastatic process by degrad-ing basement membranes (Arii et al., 1996; Klein et al., 2004). MMPs and their endogenous inhibitors participate in the inva-sive process of human osteosarcoma (Kahari and Saarialho-Kere, 1999; Kimura et al., 2011). MMPs can also promote tu-mor growth by increasing the bioavailability of growth factors in the ECM (Chambers and Matrisian, 1997). Recent studies have also suggested that anti-inflammatory agents exert sub-stantial protective effects on tumor promotion and metastasis by blocking the expression of MMPs (Kim et al., 2009). Thus, MMPs play a critical role in cancer and inflammation. In ad-dition, among the molecules involved in inflammation, reac-tive oxygen species (ROS), such as superoxide and hydrogen peroxide, likely play a role in linking inflammation to carcino-genesis. ROS are well known as key inducers of MMPs and subsequent tumor progression (Mori et al., 2004; Nelson and Melendez, 2004). These findings suggest that downregulation of ROS signals may be an effective tool for the treatment of osteosarcoma. However, the importance of ROS signals on the metastatic property of osteosarcoma cells has not been stud-ied.
A natural peptide from the enzymatic hydrolysates of the seahorse
>
Preparation of the anti-inflammatory peptide
The anti-inflammatory seahorse peptide was prepared as reported by Ryu et al. (2010). To prepare the peptide from seahorse protein, enzymatic hydrolysis using several commer-cial enzymes (alcalase, neutrase, papain, pepsin, pronase E, and trypsin) was performed. At an enzyme/substrate ratio of 1/100 (w/w), the reaction mixture was incubated for 8 h with stirring and then heated in a boiling water bath (100℃) for 10 min to inactivate the enzyme. Pronase E-derived seahorse hydrolysates exhibiting the greatest downregulation of colla-gen release and iNOS were further purified by Hiprep 16/10 DEAE FF anion-exchange chromatography (GE Healthcare, Piscataway, NJ, USA) and reverse-phase high-performance liquid chromatography using a Primesphere 10C18 (20 × 250 mm) column. The purity of the peptide was over 99% accord-ing to assessment by reverse phase-high performance liquid chromatography and N-terminal sequence analysis. The ami-no acid sequence of the purified peptide was determined to be LEDPFDKDDWDNWK by electrospray ionization mass spectrometry spectroscopy.
MG-63 human osteosarcoma cells from the American Type Culture Collection (ATCC, Manassas, VA, USA) were routinely grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bo-vine serum (FBS; Gibco-BRL Life Technologies, Grand Is-land, NY, USA), 100 U/mL penicillin, and 100 mg/mL strep-tomycin in 5% CO2-containing air at 37℃. For experiments, cells were passaged at least three times and detached with Trypsin-EDTA. Matrigel was obtained from BD Biosciences (San Jose, CA, USA). Antibodies against MMP1, MMP2, Rac1, and actin were obtained from Santa Cruz Biotechnol-ogy (Santa Cruz, CA, USA), BD Biosciences, Cell Signaling Technology (Beverly, MA, USA), and Sigma (St. Louis, MO, USA), respectively. Dichlorofluorescein diacetate (DCF-DA) was acquired from Molecular Probes (Eugene, OR, USA). Chemicals and reagents were purchased form Sigma, if not otherwise noted.
MG-63 cells were seeded in 96-well plates at a density of 1 × 103 cells/well in DMEM containing 10% FBS. Twenty-four hours after seeding, the medium was changed to serum-free DMEM, and the cells were incubated with various concentra-tions (0-0.5 mg/mL) of peptide for 24 h. Thereafter, the medi-um was carefully removed, and 100 μL of 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 1 mg/mL final concentration) solution was added to each well prior to incubation for another 3 h at 37℃ in 5% CO2. The absorbance was then measured in a microplate reader (iMark; Bio-Rad, Hercules, CA, USA) at 540 nm.
For the cell invasion assay, the undersurface of the porous membranes in the Matrigel Invasion Chambers (BD Biosci-ences) were coated with fibronectin (25 μg/mL) at room tem-perature for 1 h and washed three times in DMEM. DMEM was added to the lower compartment of the chamber. Cells were cultured in DMEM without FBS overnight and treated with or without 12-
DCF-DA was used to evaluate the generation of intracel-lular ROS. Cells (3.3 × 104 cells/well) in 24-well plates were first incubated with TPA for 24 h in the presence or absence of peptide. The cells were then washed with PBS and incubated with 10 μM DCF-DA for 30 min at room temperature. Flu-orescence was measured using a fluorescence plate reader.
>
Small GTPase Rac1 activity assay
GST-PAK-CRIB fusion protein was expressed as described previously (Miki et al., 2000) and immobilized on glutathione-sepharose beads (Amersham Biosciences, Piscataway, NJ, USA). Cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1% NP-40, 10% glycerol, 200 mM NaCl, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mM di-thiothreitol (DTT), and 1 mM phenylmethanesulfonyl fluoride (PMSF). An aliquot of 5-10 μL of cell lysate was subjected to Western blotting. Then 1 mL of cell lysate was mixed with 50 μL (bed volume) of GST-PAK CRIB beads and rotated at 4℃ for 40 min. The beads were washed three times with cold wash buffer containing 25 mM Tris-HCl (pH 7.5), 30 mM MgCl2, 40 mM NaCl, 1% NP-40, 1 mM DTT, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mM PMSF, and 20 μL of sodium dodecyl sulfate (SDS) sample buffer containing 50 mM Tris-HCl (pH 6.8), 2% SDS, 6% 2-mercaptoethanol, 10% glycerol, and 0.5 mg/mL bromophenol blue. Samples were separated by SDS-gel electrophoresis, and Rac1-GTP was detected by Western blotting.
>
Reverse transcription-polymerization chain reac-tion (RT-PCR)
Cells treated with TPA and/or peptide for 24 h were washed with ice-cold PBS twice. Total RNA was extracted using a commercial kit (RNeasy Mini Kit; Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. cDNA synthesis was carried out using 3 μg of total RNA. The fol-lowing primers were used to determine target gene levels. MMP1 (sense 5′-GGTCTCTGAGGGTCAAGCAG-3′ and antisense 5′-AGTTCATGAGCTGCAACACG-3′), MMP2 (sense 5′-ATGACAGCTGCACCACTGAG-3′ and antisense 5′-ATTTGTTGCCCAGGAAAGTG-3′), COX-2 (sense 5′-TGAGCATCTACGGTTTGCTG-3′ and antisense 5′-TGCTTGTCTGGAACAACTGC-3′), and GAPDH (sense 5′-GAGTCAACGGATTTGGTCGT-3′ and antisense 5′-TTGATTTTGGAGGGATCTCG-3′).
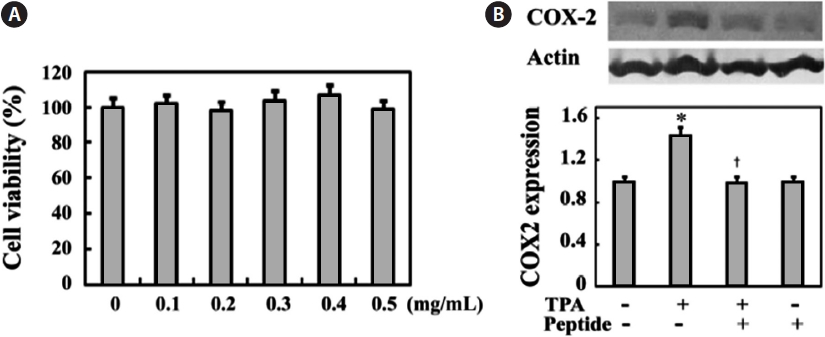
We first examined peptide cytotoxicity to human osteosar-coma MG-63 cells and determined the proper peptide con-centration showing anti-inflammatory effects in MG-63 cells. MG-63 cells were treated with 0-0.5 mg/mL peptide for 24 h. Peptide treatment at these concentrations did not signifi-cantly inhibit cell growth (Fig. 1A). In addition, Fig. 1B shows that cell stimulation with 10 ng/mL of the pro-inflammatory agent TPA induces increased expression of cyclooxygenase-2 (COX-2), a key enzyme that stimulates prostaglandin produc-tion, whereas 0.1 mg/mL peptide treatment effectively sup-presses TPA-induced COX-2 expression. This inhibitory ef-fect of peptide on TPA-induced COX-2 expression was not
significantly altered at increased peptide concentrations (data not shown). Thus, we performed all subsequent experiments using a peptide concentration of 0.1 mg/mL.
>
Peptide inhibits TPA-induced invasive migration of MG-63 osteosarcoma cells
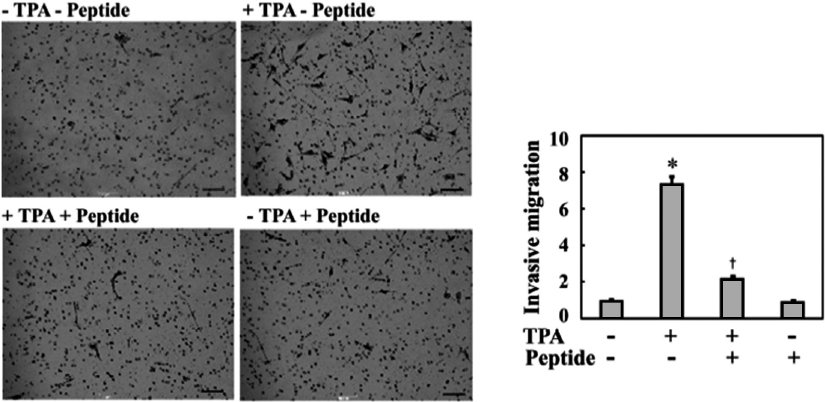
Although many recent studies have shown that anti-in-flammatory agents are capable of exerting antitumor activity in various tumor cells, their actual effects on the metastatic properties of osteosarcoma cells have not yet been described. Therefore, in this study, we investigated whether the anti-inflammatory peptide influenced the metastatic phenotype of osteosarcoma cells, such as invasive migration. As shown in Fig. 2, TPA treatment increased the invasive migration of MG-63 cells into Matrigel by approximately 7.5-fold compared to the controls, which were not treated with TPA. Treatment of MG-63 cells with 0.1 mg/mL peptide significantly inhibited TPA-induced cell invasion to approximately one-quarter of that of TPA treatment. Therefore, these results suggest that this peptide regulates an intracellular signaling cascade involved in the invasive migration of MG-63 cells.
>
Peptide decreases TPA-induced intracellular ROS generation and Rac1 activation in MG-63 cells
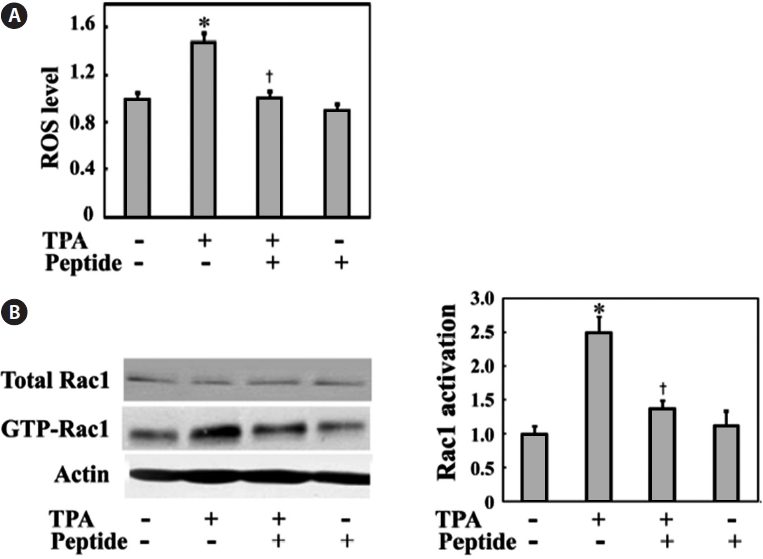
Much evidence indicates that ROS play a central role in tumor cell migration, invasion, and metastasis (Radisky et al., 2005). ROS are also well known to link inflammation to tu-mor promotion and metastasis. Thus, ROS generation might also be induced by TPA, which is also a well-known tumor promoter in MG-63 cells. We examined the intracellular ROS levels in MG-63 cells in the presence or absence of TPA stim-ulation. MG-63 cells were incubated with TPA for 24 h in the presence or absence of peptide. As shown in Fig. 3A, TPA treatment increased ROS levels to approximately 1.5-fold of those seen in the controls. Comparatively, peptide treatment successfully inhibited TPA-induced ROS generation in cells.
The changes in invasive cell migration under oxidative stress also suggest the involvement of the small GTPase Rac1, which is an upstream regulator critical for actin reorganization and invasive cell migration. Rac1 is also an upstream enzyme in NADPH oxidase-dependent ROS generation (Bokoch and Knaus, 2003). Generation of ROS under a variety of physi-ological conditions is associated with Rac1 activation (Werner and Werb, 2002; Nimnual et al., 2003). Therefore, we tested endogenous Rac1 activation in the presence or absence of TPA and peptide treatment via a GST-PAK binding assay using cell lysates. TPA treatment induced Rac1 activation, whereas pep-tide treatment decreased TPA-induced Rac1 activation (Fig. 3B). These data suggest that the TPA-induced Rac1-ROS cascade is involved in the invasive migration of these MG-63 cells.
>
Peptide reduces TPA-induced expression of MMPs in MG-63 cells
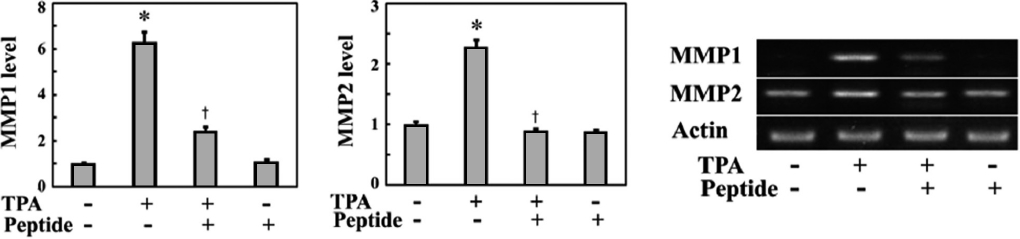
MMPs are responsible for invasive cell migration via their ECM-degrading activity. The Rac1-ROS pathway induces the expression of MMPs in a variety of cell types (Nelson and Melendez, 2004; Radisky et al., 2005). Therefore, we exam-ined whether TPA and peptide treatment affected the expres-sion of MMPs. MG-63 cells were stimulated with TPA for 24 h in the presence or absence of peptides, and then MMP ex-
pression was examined by mRNA quantification via RT-PCR. As shown in Fig. 4, TPA stimulation increased the expression levels of MMP1 and MMP2 mRNA in MG-63 cells, while peptide treatment inhibited the TPA-induced expression of these MMPs. In particular, MMP1 mRNA levels increased over 6.2-fold after TPA treatment and were significantly in-hibited by peptide treatment. These findings suggest that the peptide suppresses invasive migration of MG-63 cells by in-hibiting MMP expression through downregulating intracellu-lar Rac1-ROS signaling.
Osteosarcoma is an aggressive malignant bone disorder with a high potential to invade and metastasize. In the present study, we examined the effects of an anti-inflammatory and anti-oxidative agent on the metastatic invasive potential of MG-63 osteosarcoma cells. TPA stimulation increases the in-vasive migration of MG-63 cells, which correlates with Rac1 activation and increased intracellular ROS levels as well as increased MMP1 and MMP2 expression. In contrast, an anti-inflammatory peptide isolated from seahorse enzyme extracts attenuated TPA-induced intracellular ROS levels and Rac1 ac-tivation. The peptide also decreased the expression of MMP1 and MMP2. Therefore, these results suggest that the TPA-induced Rac1-ROS-linked cascade enhances invasive migra-tion of MG-63 cells by inducing MMPs, whereas the peptide decreases invasive cell migration through downregulation of MMP expression by suppressing intracellular Rac1-ROS signals. Also, anti-inflammatory and anti-oxidative agents are able to exert substantial protective effects against osteosar-coma promotion by blocking MMP expression.
MMPs have been implicated in primary and metastatic tu-mor growth and angiogenesis (Nelson et al., 2000; Coussens and Werb, 2002). Selected inhibition of MMPs represents an important strategy for prevention or treatment of cancer. Some commercially available synthetic drugs such as Batima-stat (BB-94) and Marimastat (BB-516) have been successfully used to lower MMP expression. However, improper metabo-lism, low oral bioavailability, poor solubility, side effects, and the risk of increased toxicity of synthetic drugs remain sub-stantial challenges (Thomas and Kim, 2010). Recently, great interest has been expressed in identifying a powerful, nontox-ic, and natural anti-inflammatory and anti-oxidative therapeu-tic agent from natural resources to prevent oxidative stress and reduce inflammatory responses and cancer metastasis. Marine resources are varied and vast, and have unique bioactivities because of the diverse environment. Thus, a marine resource-derived active peptide may be a promising, cost-effective, and safe approach to the prevention of tumor progression. Here, we successfully showed that a natural peptide from seahorse suppresses the MMP signaling response involved in invasive migration of MG-63 cells through the reduction of Rac1-me-diated ROS.
The relationship between inflammation and tumor progres-sion has been well documented, for example, recent studies showing that ROS contribute to cell migration and invasion in tumor cells. Classically, ROS were regarded as the host-defense molecules released by neutrophils for the destruction of exogenous pathogens. Aberrant increase in intracellular ROS results in physiological and pathological changes, such as cell cycle arrest, apoptosis, and ischemia/reperfusion in-jury (Boonstra and Post, 2004; Otani, 2004; Gourlay and Ay-scough, 2005). In fact, some compounds have been found to exert antitumor effects against osteosarcoma cells by inducing excessive ROS generation, cell growth defects, and apoptosis (Chen et al., 2012; Tian et al., 2012). However, recently, much evidence indicates that ROS generated at low concentrations play central roles in intracellular signal transduction pathways for a variety of cellular processes (Chiarugi, 2003; Aslan and Ozben, 2004; Poli et al., 2004). In particular, elevated oxi-dative stress has been detected in many types of cancer cells (Klaunig et al., 1998; Ambrosone, 2000), and the involvement of ROS signaling in tumor promotion and metastasis has been highlighted (Radisky et al., 2005). Consistent with these re-ports, we have shown that activated Rac1-ROS signaling in response to TPA stimulation successfully increases MMP ex-pression and results in invasive migration of osteosarcoma MG-63 cells. This is the first report to show a possible mecha-nism of the antitumor effect of an anti-oxidative agent against the metastatic property of osteosarcoma cells.
We also found that treatment of MG-63 cells with Go6983, a selective protein kinase C (PKC) inhibitor, reduces expres-sion of MMP1 and invasive migration of MG-63 cells (data not shown). The cellular receptor of TPA, relevant to tumor progression and metastasis, was found to be PKC (Debidda et al., 2003; Woo et al., 2004). ROS can be generated by TPA in a PKC-dependent manner (Datta et al., 2000). Therefore, the TPA/PKC-mediated signaling cascade likely cross talks with the MMP pathway to mediate TPA-induced invasive migra-tion of MG-63 cells via ROS generation. In addition, when we compared the seahorse peptide sequences with known se-quences in the translated GenBank database, the peptide se-quence was found to have a high identity (>90%) with the corresponding regions of α-enolase from various sources. α-Enolase is a surface receptor for plasminogen. α-Enolase-bound plasminogen promotes tumor cell invasion and cancer metastasis through conversion to plasmin and consequent ECM degradation (Pancholi and Fichetti, 1998; Vanegas et al., 2007). ROS-generating agents have been proposed to upregu-late plasminogen activator (Kiguchi et al., 2001). Plasmin also induces matrix degradation via activation of MMPs. There-fore, some other invasion-related genes, such as α-enolase and plasminogen, may also be coupled with the TPA-induced Rac1-ROS cascade to regulate MMP expression and mediate invasive migration of MG-63 cells. Additionally, the seahorse peptide is an aspartic acid-rich oligopeptide. Acidic residues are able to form ionic bonds with target molecules and also function as hydrogen bond acceptors. Many proteins that bind metal ions for structural or functional purposes possess aspar-tate or glutamate side chains as metal-binding sites. Therefore, identification of the direct binding targets of the peptide in MG-63 cells may help to precisely understand how the peptide exerts its antitumor effect in MG-63 cells and is an important subject for further research.