Survival, successful development, and distribution of kelp are influenced by environmental factors (Dayton 1985), which can affect the microscopic stages (spores, gametophyte, and male and female gametes) and the macroscopic stage (sporophyte). All life stages exhibit varying tolerances to environmental factors (Luning 1980, Deysher and Dean 1986, Amsler and Neushul 1991, Dring et al. 1996, Edwards 2000), making some species and life stages more or less adapted to changing conditions. In particular, spores will preferentially settle onto suitable substrata in appropriate microenvironments (Amsler et al. 1992, 1999). Typically, kelp spores are viable immediately upon release and may settle on the seafloor within minutes (Santelices 1990, Reed et al. 1992). The successful settlment and attachment of spores plays an important role in determining patterns of adult kelp distribution and abundance (Dayton 1985, Schiel and Foster 1986, Santelices 1990). Sedimentation has been shown to be a particularly influential parameter affecting spore survival and attachment success (Devinny and Volse 1978, Airoldi 2003).
Increasing temperatures have led to accelerated glacial melting in Alaska (VanLooy et al. 2006), which probably increases the amount of glacially-discharged sediments that enters the nearshore environment. Spores within the water column can attach to suspended particles discharged by glacial and terrestrial runoff, preventing them from attaching to bottom substrate (Arakawa and Matsuike 1992, Arakawa 2005). Sediment can also occlude suitable surfaces preventing settlement, and sediments can smother settled spores, leading to mortality (Devinny and Volse 1978, Airoldi 2003). For example, the effects of sediments on Macrocystis pyrifera (Linnaeus) C. Agardh spores appear to be immediate and significantly hindered spore attachment in the laboratory (Devinny and Volse 1978). By determining how sedimentation influences the establishment and survival of various canopy-forming kelp species, we may be able to predict how changes in this climate-change related variable may impact the kelp composition and distribution of nearshore kelp forests.
Within the nearshore of much of Alaska’s rocky coastline, the two surface canopy-forming kelp species, Nereocystis luetkeana (K. Mertens) Postels & Ruprecht and Eualaria fistulosa (Postels & Ruprecht) M. J. Wynne (formerly Alaria fistulosa; hereafter referred to as Nereocystis and Eualaria), may form mono-specific or mixed species kelp forests that provide very different structural habitats for associated communities (Efird and Konar unpublished data). The long, slender stipe of Nereocystis provides much less midwater structure than the extensive, submerged blade structure of Eualaria. The many apical blades of Nereocystis create considerably denser surface structure than the single Eualaria blade. Hence, the abundance or dominance of one canopy kelp species over the other may have habitat implications for associated communities. In addition, the density and distribution of these species are temporally and spatially variable (Schoch 2001). This has been well documented in Kachemak Bay, Alaska, where Eualaria was the dominant canopy-forming species in the 1970s (Lees 1976), while Nereocystis was the dominant species in the early 2000s (Chenelot 2003, Schoch and Chenelot 2004, Deiman unpublished data). Currently, Nereocystis still dominates but Eualaria is being observed with increasing frequency (personal observation). Kachemak Bay is an estuarine fjord under strong influence of glacial sediment discharge (VanLooy et al. 2006, Spurkland and Iken 2011). This high sedimentation may be a variable that prevents one or both canopy-forming species from settling and establishing in areas of high sedimentation (Schoch and Chenelot 2004), an effect that may increase with increasing glacial discharge.
It is important to separately analyze the effects of sedimentation on Nereocystis and Eualaria spore attachment and survival in an effort to tease apart how this variable affects each species in a controlled laboratory setting. Understanding the effects of sediments on individual species may help us discern what drives the spatial and temporal variability of these species. The objective of this study was, therefore, to determine spore attachment success under increasing levels of sedimentation, specifically the effects of suspended sediment loads, of different quantities of settled sediments, and of sediment smothering of attached spores by known sedimentation rates. Due to Nereocystis’ current dominance in Kachemak Bay, which corresponds with the increase in sediment loading from glacial melt, it was hypothesized that Nereocystis spores would have higher survival and attachment rates under increased levels of sedimentation compared to Eualaria spores.
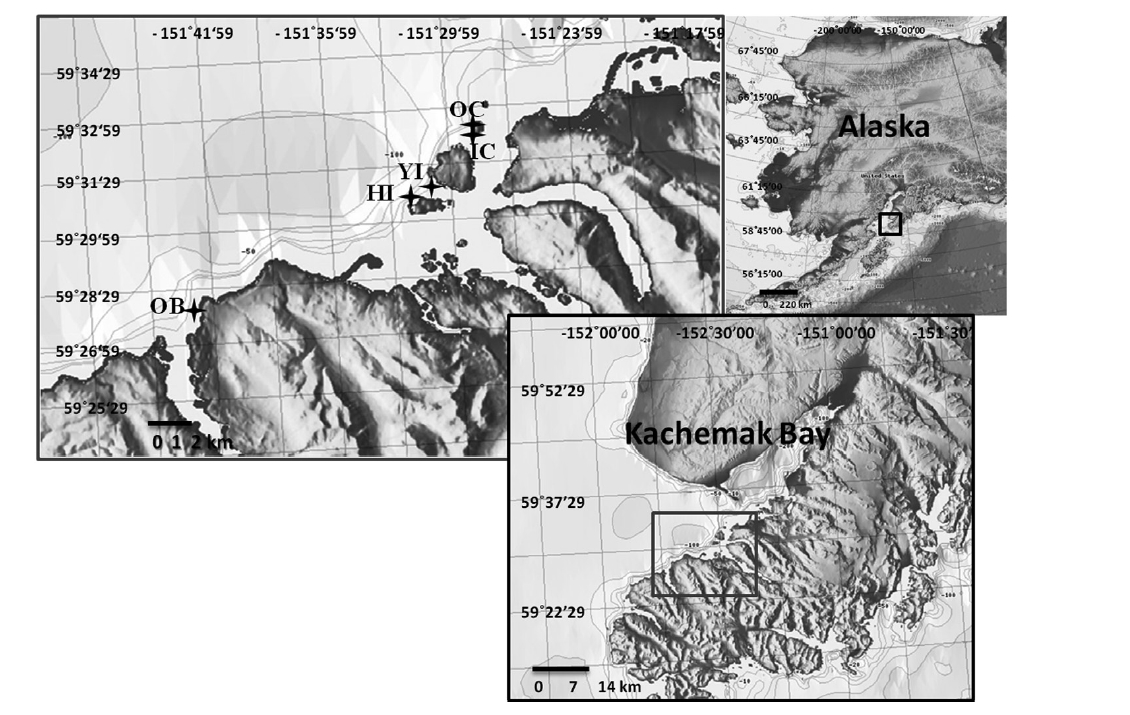
Laboratory experiments using fertile sori from two canopy forming kelp species collected at five sites in Kachemak Bay, Alaska, tested the effects of suspended and deposited sediments on spore attachment, and sedimentation on spore survival. Kachemak Bay, located north of the Gulf of Alaska at 59° N and 151° W, was chosen as the study area because of its presence of both kelp canopy-forming species, Nereocystis and Eualaria. Sorus collection sites were a representative selection of the major kelp beds along the southern shore of Kachemak Bay (Fig. 1); multiple collection sites were used to exclude any site-specific fitness patterns of the parent populations. All laboratory experiments were conducted in the summer of 2009 at the Kasitsna Bay Laboratory located on the southern shore of Kachemak Bay. Sedimentation rates in the summer within the bay range from 1 to 5 mg cm-2 day-1 (Spurkland and Iken 2011).
Sorus collections were made from individual algae growing at a water depth of 6.1 ± 1.5 m in rocky habitats at the various sites (Fig. 1). Approximately 15-20 fertile sori were collected from at least five mature individuals per species at each site. Sori of both species were collected in close proximity so that all sori at a site originated from equivalent environmental conditions. For Nereocystis, sori were gathered from floating blades at the surface
and placed into coolers filled with ambient seawater. Eualaria sori were gathered from the base of the mature sporophytes using scuba. The sori from each individual alga were placed in separate mesh bags and brought to the surface where they were immediately placed into a cooler filled with ambient seawater. At the lab, sori were kept in seawater flow-through tanks until they were used, which was no longer than 48 h after collection. For each repeat set of experiments (see below) a new batch of sori was used for both species, with each batch consisting of collections from one site.
To initiate spore release, sori from both species were gently brushed and rinsed with 0.2 μm filtered seawater to remove any macroscopic epiphytes. Nereocystis sori were then blotted dry, placed between layers of damp paper towels and newspaper, covered with plastic wrap, and placed within a cooler. The cooler was closed to keep sori in the dark and kept at 7℃ for 30 min to 4 h to condition sori for synchronized spore release (Reed 1990, Chenelot 2003). Approximately seven to ten sori were then removed, rinsed with 0.2 μm filtered seawater to remove prematurely released spores, and placed within a glass beaker filled with 1 L of 0.2 μm filtered seawater. After approximately 10 min to 1 h at 7℃ under 50 μmol photons m-2 s-1 fluorescent lighting, the Nereocystis spore solution became dark from spore and mucilage release. Eualaria sori were not incubated damp but immediately placed into beakers with 1 L of 0.2 μm filtered seawater. The Eualaria sorus solution became dark after approximately 2 to 5 h. Nereocystis and Eualaria sorus solutions were placed under the same irradiance level because previous work showed that irradiance levels up to 100 μmol photons m-2 s-1 do not affect spore attachment differently in both species (Deiman 2010).
For both species, the spent sori were removed and the solution was left undisturbed for 1 h to allow mucilage and other impurities to settle. Spore solutions were then filtered through four layers of cheesecloth to remove larger particles and the spore concentrations estimated with a hemocytometer. The spore concentrations ranged between 1,384,000 and 6,208,000 spores mL-1 for Nereocystis and between 256,000 and 2,512,000 spores mL-1 for Eualaria. These spore solutions were then diluted with 0.2 μm filtered seawater to a final concentration of 100,000 spores mL-1 solution.
To determine Nereocystis and Eualaria spore attachment rates under increasing sedimentation levels, three separate experiments were conducted to determine: (i) the effects of suspended particles on spore attachment; (ii) the effects of already settled sediment on spore attachment; and (iii) the smothering effect of settling sediment on settled spores. Sediments used for sediment concentration treatments (see below) during the experiments were collected from the general study area, sieved to contain fine silt (<63 μm), and sterilized with electromagnetic waves. Each sediment concentration treatment was replicated three times per experiment from the same spore batch per species, and each experiment was repeated five times per species with a spore solution from a different sorus collection site.
To test the effects of suspended particles on spore attachment, a clean glass slide was placed at the bottom of a 1 L container (78 cm² bottom surface area) filled with 0.2 μm filtered seawater. Varying sediment concentration treatments (0, 70, 170, 300, and 420 mg sediment L-1) were added to the containers and stirred until the sediment was homogenous throughout the container. These sediment concentration treatments were based on in situ measurements of sedimentation in Kachemak Bay of 1-5 mg cm-2 day-1 (Spurkland and Iken 2011), with 1 mg cm-2 day-1 representing conditions at the outer oceanic regions of the bay and 5 mg cm-2 day-1 representing conditions in the inner, glacially influenced regions of the bay. Once the sediment was thoroughly mixed in the container, concentrated spore solution was gently mixed into the container (separate for both species) so that the volume totaled 1 L of seawater at a concentration of 100,000 spores mL-1. Sediments were not actively kept in suspension after spore addition. The containers without sediment additions served as a control for count of maximum spore attachment.
For the experiments on the effects of already settled sediment on spore attachment and on the smothering effect of settling sediment on settled spores, the following modifications were implemented to the same basic set-up as above. For the experiment that tested the effects of already settled sediment on spore attachment, added sediment treatments (0, 70, 170, 300, and 420 mg L-1) were allowed to settle for one hour before stock spore solution was carefully and slowly added into separate containers for each species to a final concentration of 100,000 spores mL-1. The addition of the spore solution did not appear to disrupt the settled sediments. For the experiment that tested the smothering effect of settling sediment on already attached spores, spores at 100,000 spores mL-1 were allowed to settle onto a glass slide in the separate 1 L containers per species for one hour. Varying sediment treatments (0, 70, 170, 300, and 420 mg L-1) were then added to the containers and allowed to settle onto the slides with settled spores.
For all experiments, the slides were removed from the containers after 17 h (the average July-August day length in Kachemak Bay), gently rinsed, and fixed with Lugol’s solution to standardize attachment time. In each of the experiments and treatments for each species, the number of attached spores was counted in five haphazardly chosen, non-overlapping fields of view per slide with a Nikon Multi-Purpose microscope at a 40 × 10 magnification. Spore attachment counts from each sediment treatment were then divided by the spore counts from the sediment-free containers (control treatment) in each experiment per species to determine the attachment ratio. Mean counts from each experiment (n = 5) were then averaged and the standard error calculated. After the completion of all experiments, the data were tested for homogeneity of variance with a Levene’s test and normality of the data was assessed with graphical interpretation of the residuals. Data were square-root transformed to meet the homoscedasticity assumption. ANOVAs were performed to compare attachment success among sediment treatments within each experiment for each species. To compare attachment density between species, an ANCOVA was performed for each experiment with sediment treatments as covariate. Statistical significance of difference amongst the means was set at α = 0.05. An exponential regression model was used to create a regression least squares fit through the data assessing the effects of increasing sediment loads on spore attachment rates for each experiment (SAS Institute Inc., Cary, NC, USA).
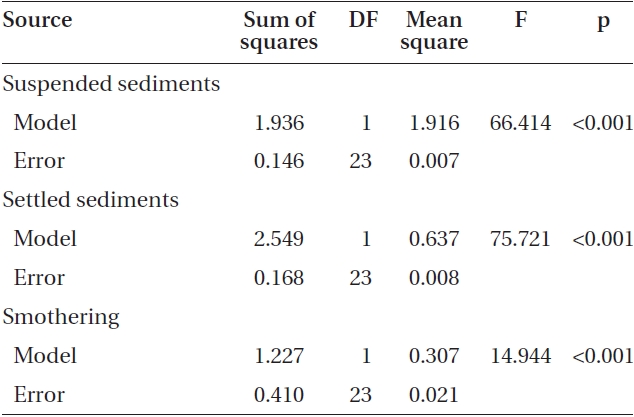
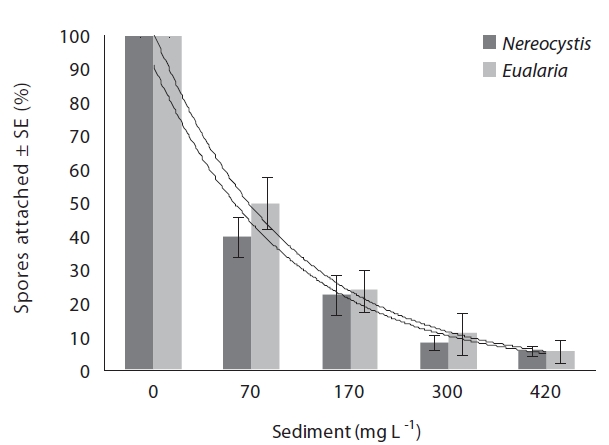
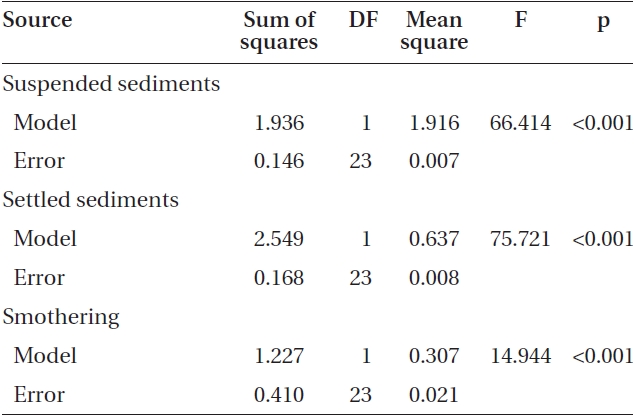
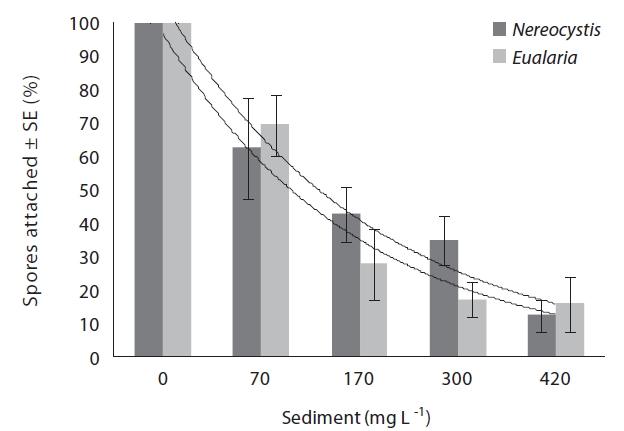
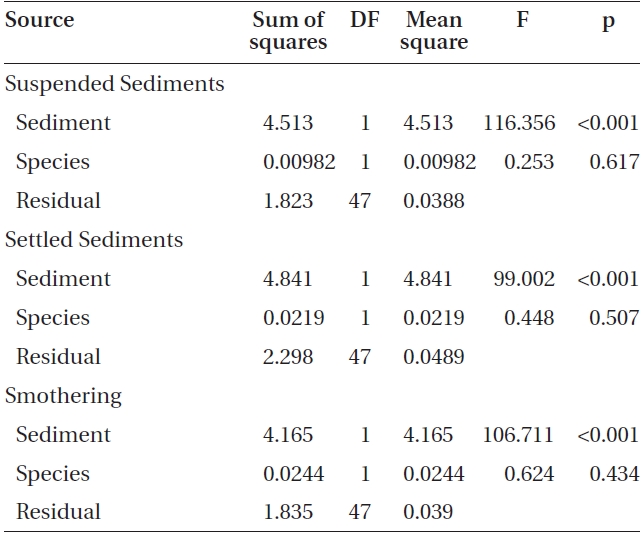
Varying sediment treatments (0, 70, 170, 300, 420 mg sediment L-1) had a significant effect on spore attachment for both Nereocystis and Eualaria in all experiments. The average Nereocystis spore attachment densities were significantly different among treatments in the experiments with suspended sediment (ANOVA, F1,24 = 66.41, p < 0.05) (Table 1). From the attachment densities in controls (0 mg sediment L-1) set as 100%, spore attachment exponentially decreased (exponential regression correlation coefficient: r2 = 0.971) to 5.8 ± 0.6% under the highest sediment load (420 mg sediment L-1) (Fig. 2). The average number of Eualaria attached spores also was significantly different (ANOVA, F1,24 = 36.73, p < 0.05) (Table 2) among the treatments of the suspended sediment experiment (Fig. 2), with an exponential decrease (r2 = 0.920) of attachment density down to 5.7 ± 1.4% at 420 mg sediment
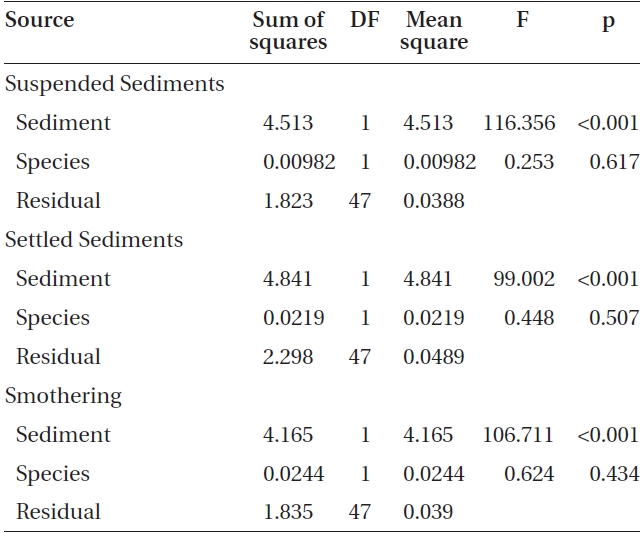
L-1 (Fig. 2). Spore attachment was not significantly different between Nereocystis and Eualaria under the various suspended sediment level treatments (ANCOVA, F1,47 = 0.25, p = 0.62) (Table 3).
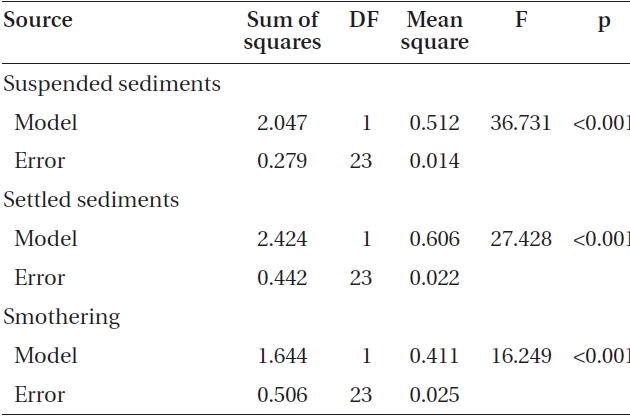
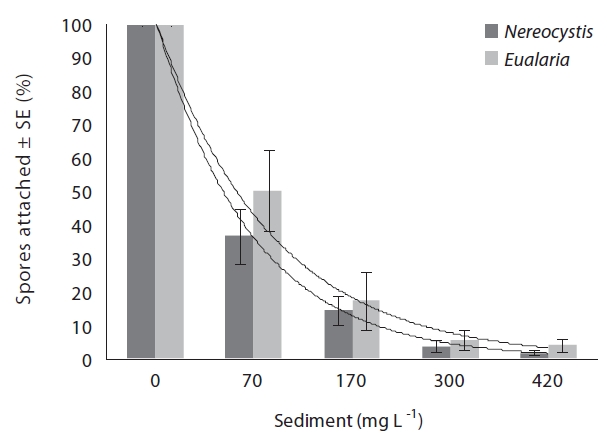
The average attachment densities for Nereocystis spores onto already settled sediments were significantly different (ANOVA, F1,24 = 75.72, p < 0.05) (Table 1) among sediment treatments, with attachment density decreasing exponentially with increasing sediment loads (r2 = 0.925) (Fig. 3). The lowest attachment densities were
found at the 420 mg sediment L-1 treatment (1.3 ± 0.3 %). Eualaria also showed a significant exponential decrease (ANOVA, F1,24 = 27.43, p < 0.05, r2 = 0.873) (Table 2) in spore attachment under increasing settled sediment levels to as low as 4.1 ± 0.8% at 420 mg sediment L-1 (Fig. 3). Again, spore attachment onto sediment-covered substrates was not significantly different between the two algal species (ANCOVA, F1,47 = 0.45, p = 0.51) (Table 3).
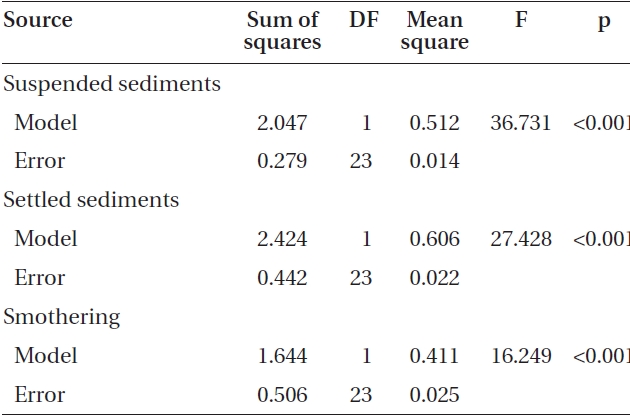
Adding sediment to previously settled spores caused a significant number of spores to be removed from the
slides for both Nereocystis (ANOVA, F1,24 = 14.94, p < 0.05) (Table 1) and Eualaria (ANOVA, F1,24 = 16.25, p < 0.05) (Table 2) compared to spore densities on slides without added sediments. The number of spores remaining on the slides for Nereocystis was lowest with 12.5 ± 1.8% at the highest sediment load of 420 mg sediment L-1 (Fig. 4). Eualaria spores showed similar results with the number of remaining spores decreasing significantly to 15.9 ± 3.2% under the 420 mg sediment L-1 treatments (Fig. 4). Percent spore removal by added sediments was not significantly different between the two algal species (ANCOVA, F1,47 = 0.62, p = 0.43) (Table 3).
In Alaska, Nereocystis and Eualaria are often found individually dominating a kelp bed although both species are locally available for settlement. Temporal and spatial distributions of Nereocystis and Eualaria have changed over the years, such as in Kachemak Bay, Alaska (Lees 1976, Schoch 2001, Schoch and Chenelot 2004), and this variablility can have effects on abundance and diversity of kelp-associated communities (Dayton 1985). It was hypothesized here that the spores of these kelp species would exhibit species-specific responses to various sedimentation rates, with Eualaria exhibiting a stronger negative response to increases in sedimentation. We observed that in a controlled laboratory setting, increases in sedimentation had detrimental effects on spore attachment density in both species. However, contrary to the hypothesis, there were no species-specific differences in the responses elicited from the two species during these experiments.
Suspended sediments significantly affected the spores of both Nereocystis and Eualaria. Sedimentation effects have been shown to constrain the establishment and development of algae through prevention of settlement and sediment spore adhesion (Devinny and Volse 1978, Arakawa 2005). Spores that have attached to suspended sediment grains are unlikely to settle successfully or survive (Arakawa and Matsuike 1992, Reed et al. 1992, Vadas et al. 1992). Similarly, while sediment covering the substratum may not prevent the spores from settling, spores attached to sediment grains are unlikely to survive to a macroscopic stage. In the current study, both algal species were similarly negatively affected by increasing suspended sediment loads, indicating no species-specific reaction to sedimentation at the levels tested. This suggests that if the amount of sediment within the water column were to increase as expected under climate change scenarios (Svendsen et al. 2002), it could have detrimental effects on the establishment of both canopy-forming species, which would decrease the available habitat for marine communities associated with these kelps.
The prevention of spore settlement by sediment covering the substratum is another negative effect on successful kelp spore attachment (Vadas et al. 1992, Reed 2000), because spores attaching to loose sediments are unlikely to survive. Nereocystis and Eualaria spores showed a similar but strong negative correlation between settlement success and increased sediment cover. Similarly, spores of the Japanese kelp, Eisenia bicyclis, showed reduced attachment responses to suspended and settled sediments as observed in our experiments (Arakawa 2005). It was hypothesized here that Nereocystis and Eualaria spores would exhibit different attachment success under analogous sediment treatments during the laboratory experiments. This may indicate that Nereocystis and Eualaria spores may have similar spore attachment mechanisms, resulting in similar responses to sediments. This could explain our laboratory results, but it does not, however, answer the ecological question of what causes the dominance of one species over the other in the field and why this has been changing over time.
Settled spores of both Nereocystis and Eualaria responded negatively to smothering by sediments during this study, with settled spores unable to remain attached to the substratum. As known for fucoids, species abundance and distribution is affected by sediments smothering already attached embryos and by scouring of settled embryos, both of which greatly impacts early life history stage recruitment and survival (Schiel et al. 2006, Irving et al. 2009). A few days of sediment burial of Durvillea antarctica and Hormosira banksii settled zygotes resulted in 100% mortality; however, settled zygotes that were several days old before sediment addition were able to survive sediment deposition and grew through the sediment layer (Schiel et al. 2006). This suggests that the effects of sediments on zygotes are immediate upon settlement but may be less significant after establishment. If these results were indicative of brown algal early life stages in general, it would support the idea that the effects on Nereocystis and Eualaria spores are immediate. Our experimental exposure of 17 h may not be long enough to show such long-term effects, but further experiments with a longer duration may show whether or not later life stages are more resilient to heavy sediment loads.
Sedimentation effects may be related to the species-specific timing of spore release. Eriksson and Johansson (2005) found that algal species with short periods of spore release benefited from a decrease in sediment deposition, while those species with a longer, continuous spore release period were more successful under heavier sedimentation rates. Here, species with long, continuos spore production and release have an advantage when they are in temporally unstable sediment environnments because spores may find a window of low sedimentation conditions over the longer time period of their release. If this were the case, a species that releases spores over long times or before times of strong sedimentation, e.g., because of runoff due to spring time melt or glacial discharge in the late summer, may have an ecological advantage over a species that releases spores more briefly during a period of heavy sedimentation. In Kachemak Bay, sedimentation rates change throughout the summer, with a slight increase in sediment levels during the month of June (National Estuarine Research Reserve System 2011, Spurkland and Iken 2011). During this study, fertile sori of Nereocystis were observed by the end of May, and no Eualaria with fertile sori were observed until nearly the end of July. It is possible that the species-specific timing of spore release relative to spring runoff or glacial sediment discharge may be one factor influencing species-specific spore success and, ultimately, kelp establishment. Sediment may be influencing species dominance, and it may be the interannual differences in sedimentation strength and timing explaining some of the temporal variability of kelp species dominance observed. Further in situ experiments and observations would greatly increase our understanding of how sediment affects algal spores.
Sediments also affect the amount of light reaching the benthos, and can thus indirectly affect spore settlement if they were responding to light. Light is important in germination and growth in other species (Edwards 2000, Santelices et al. 2002, Irving et al. 2009). For Nereocystis and Eualaria, light has not been found to be a primary mechanism influencing germination or growth of the spores of these species within the laboratory (Deiman 2010); thus, the effect of sediments reducing the amount of light reaching settled spores did not likely have a significant impact on spore success in this study. However, successful reproductive strategies, such as synchronized spore release triggered by light conditions, can ensure spore release under suitable low sediment conditions. In laboratory experiments, Nereocystis exhibited diel spore release with approximately 80% of spores being released two hours before and four hours after sunrise (Amsler and Neushul 1989). The degree of synchrony in Eualaria spore release is unknown. Spore release can also be synchronized during periods of high hydrodynamic force, e.g., in Macrocystis pyrifera (Reed et al. 1997). Simultaneously, strong hydrodynamics can also clear settled sediments from suitable substrate to allow for successful spore attachment (Ebeling et al. 1985). Synchronous release of spores during environmentally suitable conditions could give one species an advantage. Spores released in large amounts at the same time have a higher chance of settling in densities high enough for fertilization, increasing the chances that some spores may survive despite adverse conditions.
It was important to examine the tolerance of the two primary Alaskan canopy-forming kelp species to natural sedimentation rates because this variable is predicted to change over the next decade with climatic changes (Svendsen et al. 2002, VanLooy et al. 2006). Both species reacted strongly but similarly to increases in sediments, indicating that there may be other factors that allow one species to become spatially or temporally dominant. If sediment cover makes bottom substrate unavailable for settlement, spores may utilize alternative substrates. For example, Nereocystis spores can attach endophytically and epiphytically to certain red algae and develop successfully (Hubbard et al. 2004). Once Nereocystis begins to grow, it will overcome small red algae and attach to the hard rocky substrate. It is unknown if Eualaria spores have the capability to attach to other algae, but large (~3-6 m in length) Eualaria sporophytes have been observed growing on the kelp Saccharina groenlandica (Rosenvinge) C. E. Lane, C. Mayes, Druehl & G. W. Saunders in Kachemak Bay (personal observation). Settling on other algae would also result in the kelp species being removed from the substrate, which may be a means to avoid sedimentation and sediment-covered substrates. However, attaching to a biological substrate without the ability to reach a solid rocky substrate may make Eualaria more susceptible to be removed during adverse weather. The ability to preferentially select an alternative substrate may give Nereocystis the ability to out-compete Eualaria under conditions of high sedimentation; however, this hypothesis will have to be tested. There is a clear need for the documentation of the spore release season for both species and studies on a spore’s ability and mechanisms to preferentially establish onto substrata. In addition, there may be other environmental factors that affect spore survival and success.