Supercapacitors are energy storage devices that can supply extremely high but transient power output. They are being both utilized and considered for numerous power source applications, such as auxiliary power sources for hybrid electric vehicles and short term power sources for mobile electronic devices [1-3]. Recently, electrochemically active materials and metal oxides, such as Sn, Si, cobalt oxide (Co3O4), have attracted much attention as materials for supercapacitors. Among the metal oxides, Co3O4 has been reported as a priming electrode material for supercapacitors due to its excellent electrochemical performance [4-6]. However, a large specific volume change commonly occurs in the host matrix of these metals and metal oxides during the cycling process [7]. To solve this problem, graphitic carbons with high electric conductivity have been widely used as matrices for metals and metal oxides to improve their cycle performances [8-11]. Graphene, an integral part of graphite, has attracted tremendous attention since its discovery by Geim and co-workers in 2004 [12]. It is well known that metal oxides such as RuO2, IrO2, MnO2, and NiOx can improve the electrochemical performance of carbon-based supercapacitors, as they can contribute pseudocapacitance to the total capacitance apart from the double-layer capacitance from the carbon materials [13].
In this work, we report a simple synthesis approach that involves the reduction of Cobalt (II) ions from salt solution and calcination in air to form Co3O4 nanoparticles on graphene materials. Through calcinations of different temperatures, we also searched for the optimal annealing temperature that can produce the most promising electrochemical performances.
Graphite oxide was synthesized from natural graphite (SP- 1, Bay carbon) by a modified hummer’s method. The graphite powder was added into a mixture of sulfuric acid, sodium nitrate, and potassium permanganate for the acid treatment. The oxidized and treated solution was filtered and washed with HCl (10%) and subjected to centrifugation (3600 rpm, 5 min) to remove residual graphite. To prepare Co3O4 /graphene composites, graphite oxide was added to an aqueous solution of cobalt nitrate hexahydrate (Dae Jung Chemical, Korea). The solution was sonicated and NaBH4 solution was added dropwise to the above solution. The reaction mixture was heated at 90℃ for 4 h. During this process, graphite oxide was reduced to graphene and cobalt (II) ions to cobalt nanoparticles simultaneously. The black powder precipitation was filtered, washed with distilled water and absolute alcohol several times, and dried in a vacuum oven. Finally, the samples were calcined in a muffle furnace at different temperatures for 2 h in air, and then cooled to room temperature. The resulting black powder was used for active materials and carbon black (Super-P, Alfa Aesar) and polyvinylidene fluoride (Aldrich) were mixed in a mass ratio of 85:15:5 in an n-methyl pyrrolidone solution. All electrochemical tests were done in a three-electrode system. Nickel foams coated with Co3O4/graphene composites, a platinum foil and Ag/AgCl (3M KCl, 0.196 V vs SCE, Mettrohm) served as counter and reference electrode. The measurements were carried out in 6M potassium hydroxide electrolytes. Electrochemical measurements were performed in an Iviumstat (Ivium Technologies, Netherlands).
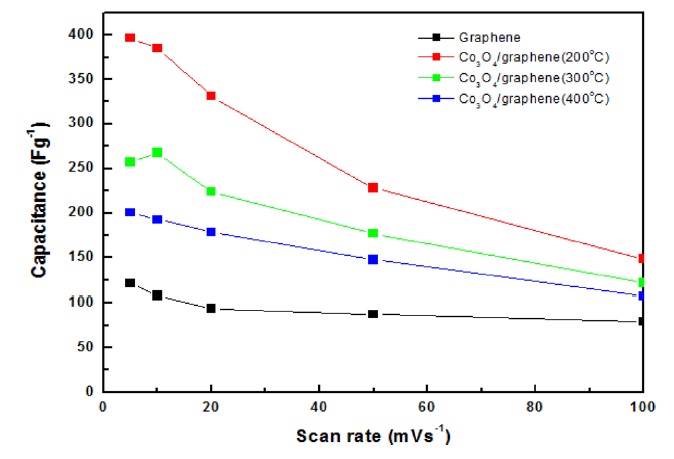
The capacitance of the Co3O4/graphene composites was compared to those of pristine graphene. The well-dispersed nanoscale Co3O4 particles on graphene not only effectively inhibited agglomeration of graphene, which resulted in a high double layer capacitance, but also increased the electrochemical active surface areas of the cobalt oxides. The capacitances of the composites that were calcinated at different temperatures are presented in Fig. 1. The capacitance of Co3O4/graphene composites was enhanced by the deposition of cobalt oxides.In particular, a sample that was annealed at a low temperature, such as 200℃, had the highest capacitance. Fig. 1 shows the variation in the specific capacitance of the as-prepared samples as a function of scan rates. The specific capacitance decreased with the increase of scan rates from 5 to 100 mVs-1. At a high scan rate (100 mVs-1), diffusion of electrolyte ions was limited due to the time constraint and only the outer active surface was utilized for charge storage. The maximum specific capacitance of the composites (200℃) was 396 Fg-1 at 5 mVs-1 and 148 Fg-1 at 100 mVs-1.
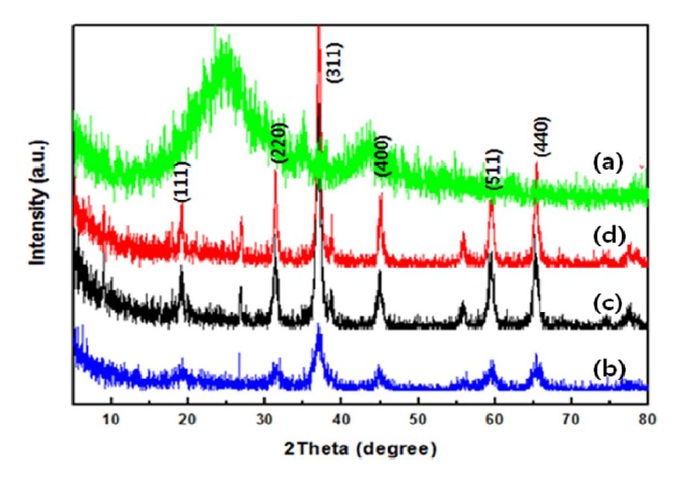
Fig. 2 shows the X-ray diffraction (XRD) patterns of Co3O4/ graphene obtained at different temperatures of 200, 300, and 400℃ for 2 h in air. The crystallinity of the cobalt oxide appeared sharply according to the increase of calcination temperature. By analyzing the peak width of the XRD patterns using Scherrer’s equation, particle sizes of the Co3O4/gra-
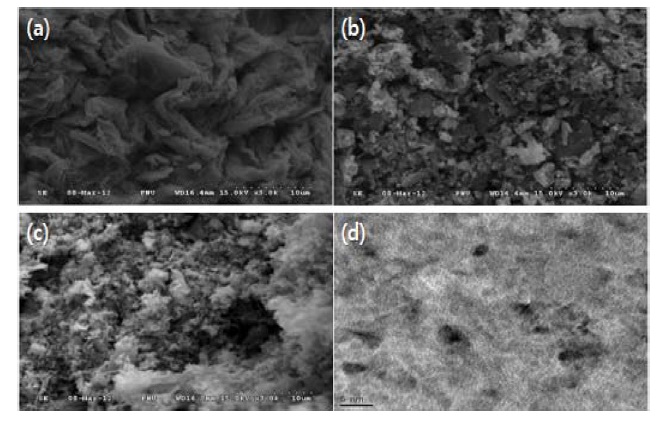
phene-200, Co3O4/graphene-300, Co3O4/graphene-400 were estimated to be 7.2, 10.3, 12.2 nm, respectively. The results indicated that the capacitances of the samples depended on the crystallinity and size of the particles. Generally graphene materials have a property that is stacked in a dry atmosphere [14]. Agglomeration of graphene sheets affects the initial capacitance because of a decrease in the active specific surface areas that include a double layer capacitance. Fig. 3 shows the transmission electron microscopy and scanning electron microscopy images of Co3O4/graphene composites at different annealing temperatures. Annealing Co3O4/graphene samples in air at different temperatures significanty changed the morphology of the composites (Figs. 3a-c). The surfaces of the composites were softened and were generated as a flower- like morphology depending on the increase of calcination temperatures. In addition, the Co3O4 particles with an average size of 7 nm were dispersed homogeneously on the surface of the graphene (200℃). A sample annealed at 200℃ was found to be appropriate in terms of the capacitances. These structures had an enhanced specific surface area and electrolyte ion intercalation spots during an electrochemical reaction.
In conclusion, Co3O4/Graphene composites were prepared using a simple chemical approachusing different calcination treatments. Co3O4/graphene composites obtained by annealing at 200℃ showed a high specific capacitance of 396 Fg-1 at 5 mVs-1. The morphology and particle size of the cobalt oxide were tunable through the annealing process. The enhanced capacitance for the composite electrodes was related to the combination effect by the Co3O4 pseudo-capacitance and electrical double layer capacitance of the graphene. Co3O4 particles on the graphene provided electrochemical reaction sites and ion intercalation spots that were accessible to electrolytes ion.