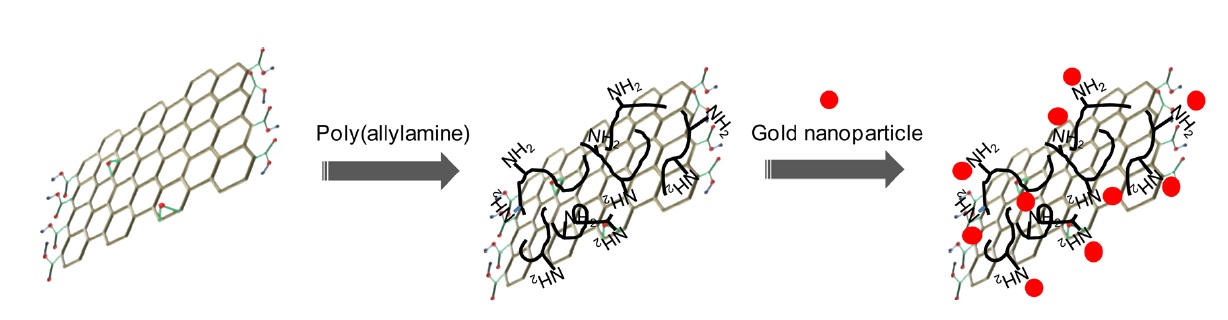
Graphene has been considered as an emerging carbon allotrope on account of its unique chemical structure, which consists of a single-layer sp2-hybridized carbon atom network that is also compacted in a perfect honeycomb lattice [1]. This unique chemical structure gives graphene excellent electrical, mechanical and optical properties, thus attracting much commercial and academic research interest [2]. Therefore, many synthetic strategies for graphene have been developed to make large quantities of high-quality graphene in a cost effective manner. For the synthesis of high-quality graphene, epitaxial growth and chemical vapor deposition methods are generally employed, but these methods are not compatible with large-scale synthesis in solution-processible form, Moreover, they require expensive high-temperature processes [3,4]. In this regard, chemical oxidation and reduction methods have been considered as promising approaches for the graphene synthesis from graphene oxide (G-O) [5]. However, strong and toxic reduction agents and surfactants are essential to reduce G-O fully in an aqueous phase [6]. To solve this problem, many studies have attempted to develop a new aqueous and environmentally friendly reduction strategy using bacterial respiration [7], potassium hydroxide (KOH) [8], polyvinylpyrrolidone [9], ascorbic acid [10], sugar [11], and protein [12]. Among the non-toxic reduction agents, protein has significant advantages because adsorbed proteins can be harnessed as a universal type of glue for various nanomaterials for nanocomposite synthesis. Recently, our group reported that dextran, a natural polymer composed of saccharides, can be successfully employed as a multifunctional agent for G-O reduction and functionalization; additionally, we found that dextran-functionalized G-O (D-RG-O) can directly reduce gold salt, resulting in a D-RG-O/ gold nanocomposite [13].
In this study, polyallylamine hydrochloride (PAAH), a synthetic linear polymer containing numerous amine groups as a pendent in each repeating unit, was employed as a new multifunctional agent for G-O reduction and functionalization in an aqueous phase. The PAAH-functionalization of G-O proceeds through the covalent linkage formation between many amine groups of PAAH and epoxy groups of G-O under a basic condition [14]. In this strategy, amine groups and the basic condition are critical because the basic condition and organic amine compounds can induce a reduction and chemical conjugation reaction [8,15]. The synthesized PAAH-functionalized G-O (PAAH-G-O) is composed of numerous amine groups on its surface and is readily dispersed in water due to the electrostatic repulsion from the positively charged amine groups. It is feasible for use in the synthesis of PAAH-G-O/gold nanocomposites [16].
Graphite (FP 99.95% pure) was purchased from Graphit Kropfmuhl AG (Hauzenberg, Germany). Sodium nitrate, sodium sulphate, potassium hydroxide, trisodium citrate dehydrate and hydrogen peroxide (30% in water) were purchased from Junsei (Japan). 3-aminopropytriethoxysilane (3-APTES), 3-glycidylpropyltrimethoxysilane (3-GPTMS), potassium permanganate, anhydrous toluene, PAAH (MW 15 000) and sodium borohydride were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). Hydrogen tetrachloroaurate (III) hydrate was purchased from Kojima Chemicals Co. (Sayama, Saitama, Japan). Nitric acid and sulfuric acid were purchased from Samchun (Seoul, Korea). Ethanol was purchased from Merck (Darmstadt, Germany). 500-nm SiO2/P++ Si substrates (500 μm in thickness) was purchased from STC (Japan). All chemicals were used as received.
2.2. Preparation of graphite oxide (GO) powder
Three gram of graphite and 1 g of sodium nitrate were added to 46 mL of sulfuric acid while stirring in an ice bath. Then, 6 g of potassium permanganate was gradually added to the graphite mixture and stirred below 20℃. After this addition, the temperature of the graphite mixture was raised to 35℃ and the mixture was stirred for an hour. 40 mL of water was added to the graphite mixture and this was stirred for 30 min and diluted with 100 mL of water in an ice bath to prevent rapid boiling up to 95℃. Finally, 6 mL of hydrogen peroxide (30%) was slowly added to the mixture, causing a color change to bright yellow from dark brown. The final mixture was filtered with filter paper (Number 3, Whatman) and washed with an abundance of water until the filtrate was neutralized. The solid on the filter paper was dried under reduced pressure for 48 h.
2.3. G-O reduction and functionalization with PAAH
Twenty mg of GO was dispersed in 100 mL of water containing 80 mg of PAAH and 100 mg of KOH by sonication for 1 h. The suspension was vigorously stirred at 70℃ for 24 h. After cooling to room temperature, the PAAH-G-O was purified and collected by centrifugation at 8228 relative centrifugal force (rcf) for 30 min and re-suspended in water by sonication. The centrifugation and re-suspension processes were repeated three times.
2.4. Preparation of gold nanoparticles
Twenty mL of 0.25 mM HAuCl4 and 0.25 mM tri-sodium citrate were prepared in a conical tube, after which 0.6 mL of 0.1 M NaBH4 (ice-cooled) was added to the solution at once under gentle shaking. This gold nanoparticle suspension was used after 2 h from the synthesis.
2.5. Preparation of PAAH-G-O/gold nanocomposite
Ten mL of PAAH-G-O suspension (0.5 mg/mL) was mixed with 10 mL of the prepared gold nanoparticle suspension by sonication for 30 min and then incubation for 1 h. After incubation, the mixture was centrifuged at 8228 rcf for 30 min and re-suspended in water by sonication. The centrifugation and resuspension processes were repeated three times to purify and collect the PAAH-G-O/gold nanocomposite.
All atomic force microscopy (AFM) images and line profiles were obtained with an XE-100 (Park System, Korea) with a backside gold-coated silicon SPM probe (M to N, Korea). The UV-vis spectra were recorded with a UV-2550 (Shimadzu, Japan). The diameter of the gold nanoparticles was determined by Tecnai G2 F30 field emission transmission electron microscopy (FEI Company, The Netherlands). The surface morphology of the PAAH-G-O/gold nanocomposite was observed by an S-4800 field emission scanning electron microscopy (SEM; Hitachi, Japan). Raman characterization was performed by LabRAM HR UV/vis/NIR (Horiba Jobin Yvon, France) using an Ar ion CW laser (514.5 nm) as an excitation source focused through a BXFM confocal microscope equipped with an objective lens (50×, numerical aperture = 0.50). Fourier transform infrared (FT-IR) spectra measurements of the GO powder were performed with an EQUINOX55 (Bruker, Germany) using the KBr pellet method.
The scheme of the synthetic processes for the G-O, PAAHG- O and PAAH-G-O/gold nanocomposite is shown in Scheme 1. The GO was synthesized by the modified Hummers’s method and exfoliated in water by sonication [17]. It is well known that G-O presents a negative charge on its surface owing to the abundant oxygen-containing functional groups, which are also reactive sites for chemical conjugations. In our system, the epoxy groups of the G-O sheets were expected to react with the amine groups of PAAH in the presence of KOH; at the same time, the reduction of G-O can occur due to the presence of both the amine group and KOH.
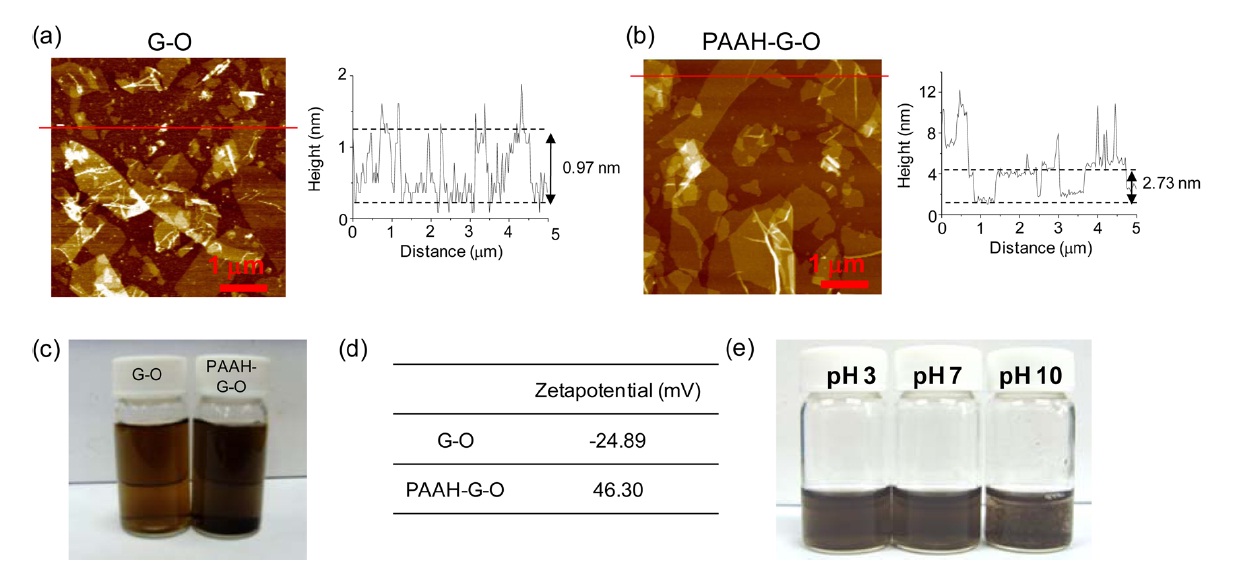
The exfoliation of GO into single sheets in water was confirmed by an AFM analysis of G-O sheets adsorbed on a 3-APTES treated silicon substrate. The thickness change of the G-O sheets induced by PAAH functionalization was also confirmed by an AFM analysis of PAAH-G-O sheets adsorbed onto a 3-GPTMS treated silicon substrate. The thickness of the G-O
sheets was about 0.97 nm, similar to the reported thickness of G-O single sheets [18]. This value was increased to 2.73 nm by PAAH functionalization. Because the PAAH was decorated onto both of the basal planes of the G-O sheets, the increase in the thickness of a single basal plane was thought to be about 0.88 nm (Figs. 1a and b). Next, the colloidal state of the G-O and PAAH-G-O sheets was characterized by a zeta potential analyzer (Fig. 1c). The zeta potential of G-O sheets in water had a negative value (-24.89 mV) due to the oxygen functional groups. This value became positive (46.30 mV) due to the amine groups introduced by PAAH, as expected (Fig. 1d). This result indicates that the driving force for the dispersion of PAAH-GO is electrostatic repulsion between protonated amine groups. This was confirmed by precipitation of the PAAH-G-O in the basic condition (Fig. 1e). The AFM profile, surface zeta potential changes and colloidal stability test results suggest that the G-O sheets were successfully functionalized by PAAH.
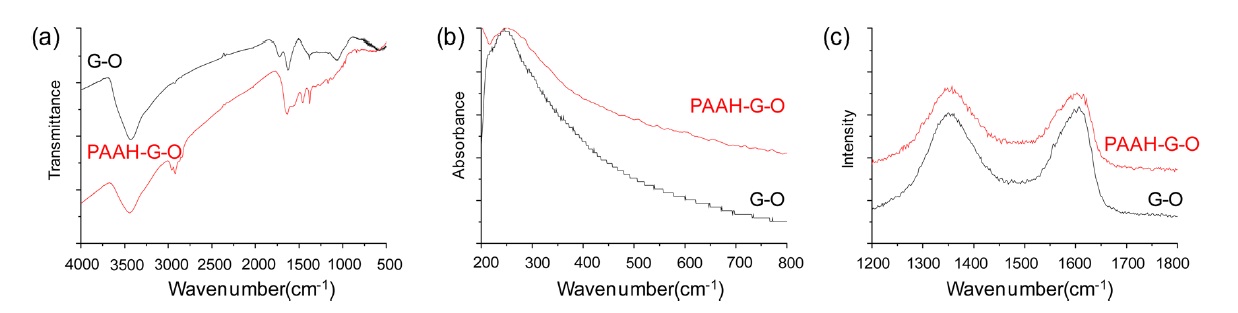
The PAAH-functionalization induced G-O reduction was monitored using various analytical tools, in this case UV-vis, Raman and FT-IR spectroscopy. The UV-vis spectrum of G-O
showed a characteristic absorption peak at 244 nm from the π-π transition of aromatic C-C bonds. After the PAAH-functionalization of the G-O sheets, the absorption peak of G-O was redshifted to 256 nm and the absorbance of the overall visible region was increased, as the PAAH-functionalization process induced the partial restoration of the sp2 carbon domain (Fig. 2a). Raman characterization also confirmed the reduction of G-O through PAAH-functionalization by showing the change of the relative D/G peak intensity ratio from 0.96 of G-O to 1.01 of PAAH-G-O (Fig. 2b) [18]. The D and G peaks respectively originated from the defect site of the graphitic structure formed during the oxidation process and from the pristine graphitic structure. Therefore, their relative ratio was a type of barometer that in this case defines the quality of the graphene crystalline structure [14]. Finally, the FT-IR spectrum of the PAAH-G-O clearly showed the dual functions of the PAAH for G-O reduction and functionalization. The following characteristic peaks of G-O appeared in the FT-IR spectrum: O-H vibration at 3432 cm-1, C=O stretching at 1729 cm-1 and C-O stretching at 1076 cm-1 with weak aliphatic C-H stretching peaks at 2854 and 2919 cm-1 (Fig. 2c). In contrast, PAAH-G-O showed weak O-H vibration and C-O stretching along with the disappearance of C=O stretching. In addition, the aliphatic C-H stretching peaks were considerably enhanced and new C-N bond stretching was noted at 1257 cm-1, which indicated the covalent conjugation of PAAH to the G-O sheets (Fig. 2c). Based on the thorough characterization, it was suc-cessfully confirmed that PAAH acted as a multifunctional agent for G-O reduction and functionalization.
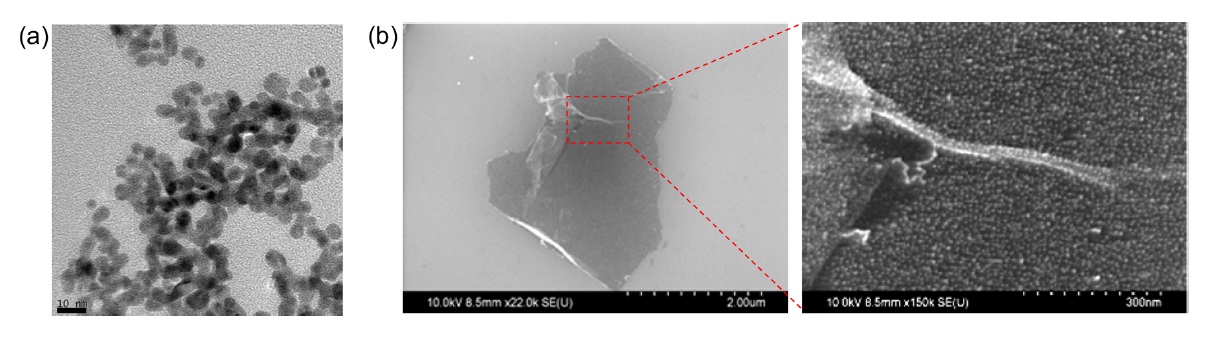
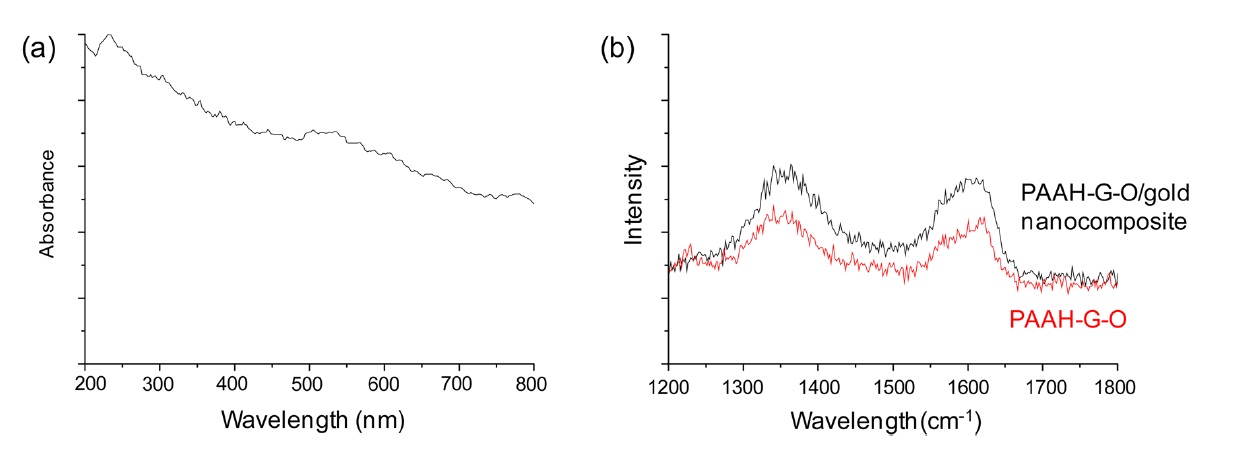
The PAAH-G-O was then harnessed as support for 5-nm gold nanoparticle immobilization for nanocomposite synthesis (Fig. 3a). Colloidal suspensions of the PAAH-G-O and gold nanoparticles were mixed by sonication for 1 h to synthesize uniform nanocomposites because the abundant amine groups of PAAHG- O had strong affinity toward gold nanoparticles due to the electrostatic interaction between the positively charged PAAHG- O and the negatively charged gold nanoparticles [19,20]. SEM images of a synthesized PAAH-G-O/gold nanocomposite showed a homogeneous distribution of 5-nm gold nanoparticles immobilized on PAAH-G-O sheets (Fig. 3b). This result is in good agreement with the UV-vis spectrum of the PAAH-G-O/ gold nanocomposite, which showed a characteristic surface plasmon resonance peak around 520 nm (Fig. 4a). In addition, the incorporation of gold nanoparticles onto PAAH-G-O sheets led to an enhancement of the Raman signal of PAAH-G-O. The D and G peaks of PAAH-G-O were respectively increased 1.30 and 1.37 fold due to the localized surface-plasmon resonance effect from the surface-incorporated gold nanoparticles (Fig. 4b).
In conclusion, functionalization and reduction of G-O by PAAH conjugation was successfully carried out under a reaction condition. The synthesized PAAH-G-O had a positively charged surface and good water dispersibility due to the plentiful amine groups. The prepared PAAH-G-O sheets were then successfully harnessed as support for gold nanoparticle immobilization. The high water dispersibility and abundant amine groups make the PAAH-G-O feasible for various biological applications, such as drug delivery, photodynamic therapy and near-IR image probe applications. We believe that this synthetic strategy is a costeffective, simple and environmentally friendly approach for the synthesis of G-O derivatives with excellent applicability to many areas in current biological research.