Electrochemical capacitors have attracted considerable research attention worldwide in recent years due to their many benefits, such as their high power density, long cycle life, short charging time, and relative safety compared to secondary batteries [1-3]. Electrochemi-cal capacitors are now used in devices such as memory back-up systems, uninterruptable power sources, and portable electronics. Currently, research is focused on improving the en-ergy density of these capacitors [4,5]. According to the energy-storage mechanism, electro-chemical capacitors can be classified as carbon-based electrochemical double-layer capaci-tors (EDLCs) [6-8] and conducting polymer [9-11] or metal-oxide-based pseudo-capacitors [12-14].
In particular, carbon materials are promising candidates for EDLCs applications owing to their chemical stability, low-cost, fine conductivity and many types of existing forms [15]. However, because the raw carbon materials have a tendency to aggregate due to their fine size and high surface energy [16], surface treatments are indispensable for improving the ef-ficiency of the specific capacitance. The characterization of carbon blacks (CBs) depends on many parameters, such as their particles size (inversely proportional to the surface area), the aggregation of the CB particles (structure, measured as absorption capacity) and the surface chemistry [17].
The goal of this work was to obtain high-performance CB electrodes for capacitor ap-plications and to determine the effects of the heat-treatment temperature on electrochemical behaviors of the heat-treated CBs. The heat-treated CBs were characterized by X-ray pho-toelectron spectroscopy (XPS) and X-ray diffraction (XRD). The electrochemical behaviors of the heat-treated CBs were also investigated by cyclic voltammetry and in galvanostatic charge-discharge experiments.
2.1. Materials and sample preparation
In the experiments here, commercial CBs were used. The CBs were heated in a tube furnace to modify the carbon surfaces and structures. They were then treated at 650℃, 850℃, 1000℃, and 1100℃ at a constant heating rate of 5℃/min under a nitro-gen flow of 200 mL/min for 5 h. The starting material was used as-received, and the samples treated by heating were denoted CB-HT650, CB-HT850, CB-HT1000, and CB-HT1100.
A surface analysis of CBs was conducted by means of XPS. XPS measurements were made with a Thermo Scientific (USA), K-Alpha device with a monochromated Al Kα X-ray source (hυ = 1486.6 eV). The base pressure in the sample chamber was maintained within in a range of 10-8 to 10 -9Torr. Survey spec-tra were recorded at O1s and C1s photoelectron peaks. All of the samples were characterized by recording their XRD patterns on a Rigaku D/MAX 2200V/PC X-Ray diffractometer (Japan) us-ing a CuKα radiation. Transmission electron microscope (TEM) images on a Jeol JEM 2100F field-emission electron microscope (Japan) were taken to observe the structural properties of the heat-treated CBs at different temperatures.
2.3. Electrochemical properties analysis
The cyclic voltammograms (CVs) and galvanometric charge-discharge curves of the CBs were examined by the IviumStat (Japan) instrument in a three-electrode cell system. A conventional three-electrode cell was formed with Ag/AgCl as the ref-erence electrode, Pt wire as the counter electrode, and a working electrode.
The active materials as the working electrodes were prepared by pressing a mixture of 90 wt.% active materials, 10 wt% poly-vinylidene fluoride binder and a small amount of N-methylpyr-rolidone solution into a Ni-form with an area of 1.5 cm2. This was followed by drying under a vacuum at 80℃ for 24 h.
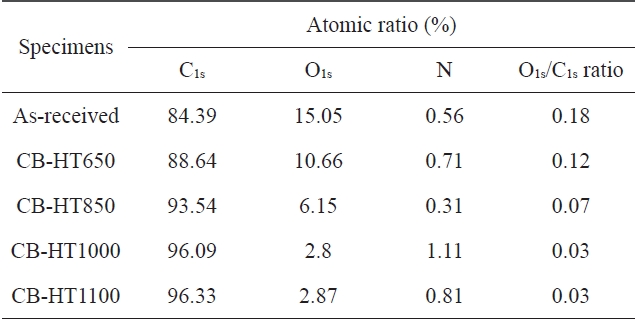
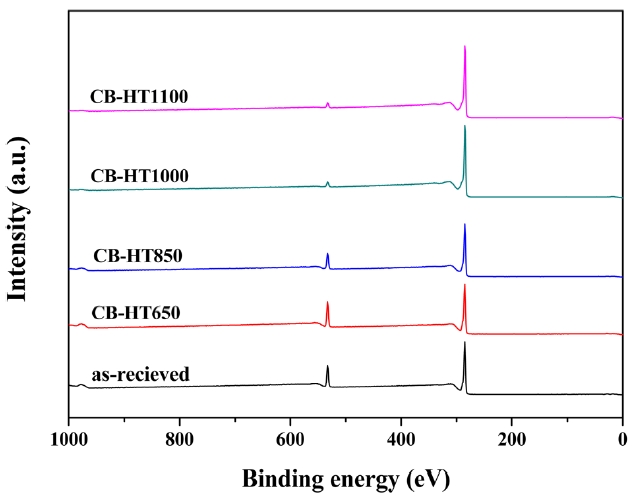
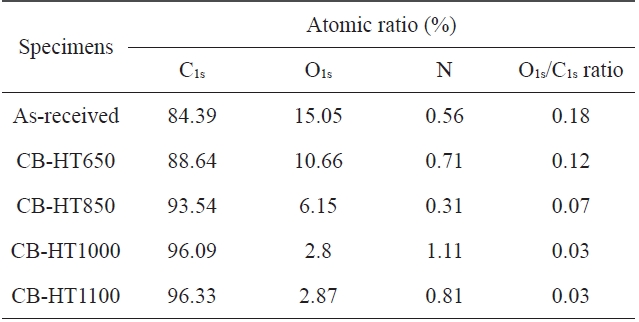
An XPS analysis was carried out to analyze the elemental compositions of the CBs treated at different temperatures. The results are summarized in Table 1. As shown in Table 1, the ratio of C1s to O1s decreased with an increase in the heat-treatment tem-perature. Fig. 1 shows that all samples exhibit nearly the same structure due to the fact that CBs, regardless the heat-treatment temperature, have a similar binding energy. The C1s and O1s peaks were centered at approximately 285 eV and 530 eV, re-spectively. The increase in C1s can be attributed to the increase in the graphitic characteristic of the CB surfaces with an increase in the heat-treatment temperature.
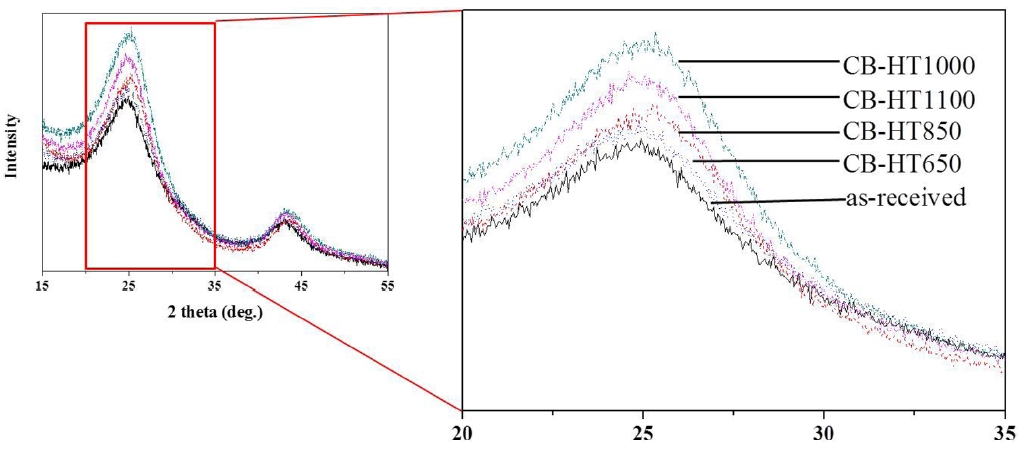
XRD patterns were used to investigate the changes in the crystalline structure caused by an increase in the heat-treatment temperature. Fig. 2 shows the XRD patterns of the heat-treated
[Table 1.] Elemental composition of the as-received and heat-treated carbon blacks
Elemental composition of the as-received and heat-treated carbon blacks
CBs treated at different temperatures. As shown in Fig. 2, the intense peak at low angle around 25° can be attributed to the 002 reflection and peak at a higher angle of around 43° can be attributed to the 100 reflection. The graphite (002) peak intensity gradually increases with the heat-treatment temperature. From these patterns, it can be confirmed that the crystallinity of CBs increases with an increase in the heat-treatment temperature.
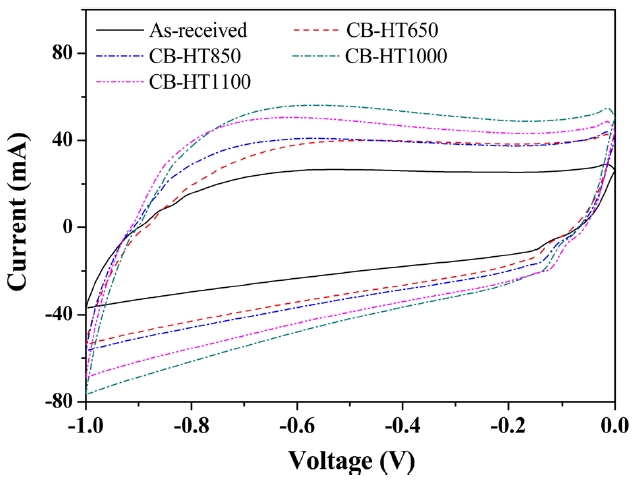
The electrochemical behaviors of the as-received and heat-treated CBs sample were investigated under identical electro-chemical conditions. The CVs of the carbon electrodes were obtained in order to monitor their current and capacitance re-sponses. Fig. 3 shows CV plots of the as-received and heattreated CBs samples at a sweep rate of 50 mV·s-1 with apotential range of -1.0 to 0.0 V vs. an Ag/AgCl reference electrodes. As shown in Fig. 3, the I-V curves exhibited slightly distorted rect-angular shapes, which is a characteristic of an electrochemical capacitor. In addition, the heat-treated CBs showed significantly better electrochemical properties than the as-received CBs.
To obtain more detailed information about the capacitance, galvanostatic charge-discharge curves were assessed at various current densities between 0 and 1 V in a further investigation of the performances of the representative CB samples. The specific capacitances of the electrodes were calculated according to the
following Eq. (1):
Here, Cm is the specific gravimetric capacitance (F g-1), w is the mass of the active material, and i is the galvanostatic current applied during the discharging times (Δ
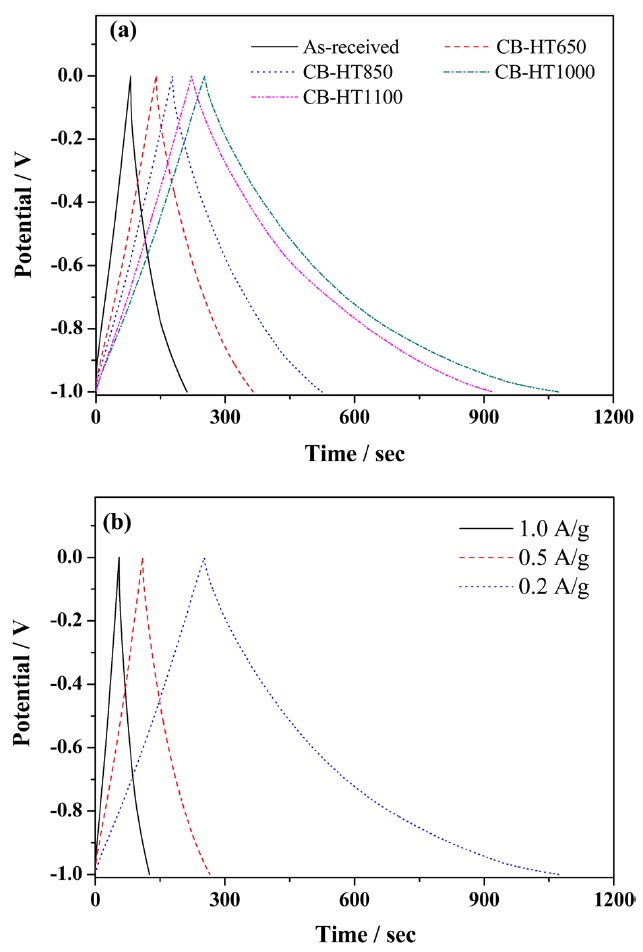
Fig.4 a shows V?t plots of all of the electrodes at a constant current density of 0.2 A?g-1, and Fig.4 b shows V?t plots of CB-HT1000 at the different current densities of 0.2, 0.5, and 1 A?g-1. The charge-discharge curves of all the electrodes obtained de-pict not only nearly triangular shapes but also a reduction in the voltage drop regions, indicating their enhanced capacitive behaviors. However, the discharge curve of the CB-HT1000 and 1100 samples have long tail shapes due to the change in the structural properties as well as the increase in the number of well-dispersed particles [18].
The specific capacitances, originally at 26 F·g-1,at a current density of 0.2 A·g-1 for the CB-HT650, CB-HT850, CB-HT1000 and CB-HT1100 samples were found to be 45.2, 69.4, 140 and 165 F·g-1, respectively. In the case of CB-HT1100 sample, the
specific capacitance decreased to 140 F·g-1. The maximum spe-cific capacitance among all specimens was noted in the CB-HT1000 sample. This can be attributed to the high crystallinity of the heat-treated CB samples at 1000°C.
To understand the increase in the specific capacitance, the charge-discharge curves of the samples with various current
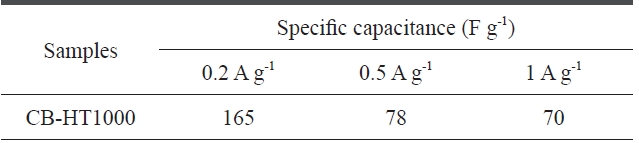
[Table 2.] Specific capacitance of CB-HT1000 at current densitiesof 0.2 0.5 and 1A? g-1
Specific capacitance of CB-HT1000 at current densitiesof 0.2 0.5 and 1A? g-1
densities in their optimal potential range were also estimated, as shown in Fig. 4b and Table 2. When the applied current density was increased from 0.2 to 1 A·g-1, the specific capacitances of CB-HT1000 remained at about 42%.
A heat treatment of CBs was performed to obtain high-perfor-mance CB electrodes for capacitor applications. The structural and electrochemical properties of the heat-treated CBs were ex-amined and several major conclusions were reached from the results.
The specific capacitance of the heat-treated CBs increased with an increase in the heat-treatment temperature. Among all of the electrodes examined here, the specific capacitance of the CB-HT1000 sample reached a maximum value of 165 F·g-1, as calculated from the charge-discharge curves. On the other hand, above a heat-treatment temperature of 1000°C, the specific ca-pacitance decreased. These results occurred because the crys-tallinity of the CBs increased as the heat-treatment temperature increased to 1000°C.