During the last two decades, interest in the preparation and utilization of carbon foams has been rapidly growing due to their advantageous properties. Carbon foams have been used as catalyst electrodes, supports, tunable thermal insulations, high temperature applications, lightweight structural parts, radar absorption materials, filters for molten metals and corrosive chemicals, etc. [1,2].
Recently, a less time-consuming process for fabricating carbon foams using organic polymers has been developed. Representative examples of polymeric materials which can be used as precursors for glassy carbon foams are phenol-formaldehyde, resorcinol-formaldehyde, phloroglucinol-formaldehyde, polyvinylidene chloride, polyacrylonitrile, furfural resin, and their blends [3]. In order to produce carbon foams using polymeric precursors, the use of a sacrificial template is an efficient approach to generate pre-determined pore sizes. The sacrificial templates are impregnated or filled into carbon precursors. After the carbonization and in-situ or subsequent template removal, macroporous carbon monoliths can be easily obtained.
As an alternative method, the polymerization-induced phase separation principle has been utilized to synthesize porous carbon monoliths. An initial homogeneous solution of reactive monomers as the carbon precursor, and non-reactive components (NRCs), undergo phase separation in the course of polymerization, to give a co-continuous morphology. In this process, ethyl glycol derivatives or polyethylene glycol have been mainly used as NRCs [4].
Room temperature ionic liquids (RTILs) are unique solvents that contain an organic cation and an inorganic anion in the liquid state at ambient condition. RTILs such as imidazolium, pyridinium, and ammonium salts have a high ionic mobility and ion concentration. In addition, RTILs have negligible vapour pressure, high thermal stability, and excellent electrical conductivity [5,6]. In this article, macroporous carbon foams were prepared using phenol-formaldehyde (PF) as a carbon precursor and alcohol-soluble ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate (BMIPF6), as pore generator. The effect of the BMIPF6 on the properties of carbon foams was investigated.
Phenol (99+ %) was purchased from Sigma-Aldrich (USA). BMIPF6 was obtained from C-tri (Korea). NaOH as a catalyst, formaldehyde (37% aqueous solution), and ethanol were supplied by Duksan Chemicals (Korea).
2.2. Preparation of macroporous carbon foams
Precursors for carbon foam were synthesized by a typical condensation polymerization technique. 3.24 g phenol was poured in 50 mL beaker. 0.9/1.8/3.6 g BMIPF6 (10/20/40 wt% to 9 g ethanol, respectively) and 0.45 g NaOH were dissolved thoroughly in 0.45 g alcohol. Temperature was elevated to 50℃ . Four point three seven gram formaldehyde (the molar ratio of P/F: 1.6) was injected into the beaker and polymerization was continued at 300 rpm for 6 h. Then temperature was elevated to 70℃ and the polymerization proceeded for an additional 36 h. The cured body was pyrolyzed stepwise in order to prevent cracks on the surface: (1) ramping the temperature up to 120℃ with a heating rate of 2℃ /min and holding for 2 h, (2) raising to 600℃ and holding for 2 h, (3) increasing to 800℃ with holding for 2 h. Then it was cooled down to room temperature. Fig. 1 shows the procedure for the preparation of the carbon foams.
Scanning electron microscope (SEM) micrographs were obtained by Hitachi S-4300. The samples were coated with platinum before characterization. Thermogravimetric analysis (TGA) was carried out using a Diamond TG/DTA Lab System (Perkin Elmer) with the heating rate of 10℃ /min. The structural properties of the carbon foams were determined by XRD (D/ MAX 2200V/PC, Rigaku, Japan) using a diffractometer with Cu Kα radiation over the 2θ range of 20-100℃ . The electrical conductivity was examined by MCP-T610 (Mitsubishi Chemical Corporation, Japan). The apparent density (ρapp), pore size distribution and porosity (?) of the carbon foams were examined by mercury porosimetry (Micromeritics Poresizer 9320). Skeletal density (ρs) was calculated based on L:
and total volume was calculated based on [7]:
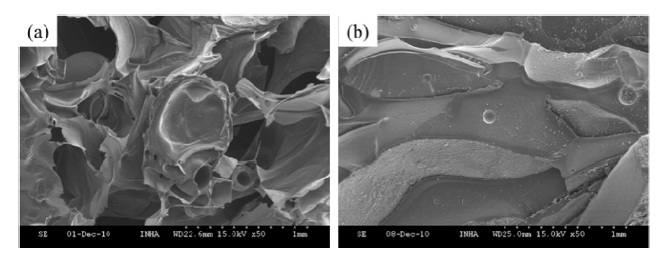
It is noted that the molar ratio of phenol (P) to formaldehyde (F) affects the resulting pore structure and pore size of the prepared porous carbon materials. A P/F molar ratio of 0.8 is a very general synthesis ratio [8]. It has been known that an 0.8 P/F molar ratio produces mesopore and micropore structures. Since we aimed to prepare macroporous carbon foams, a different P/F molar ratio was needed. Figs. 2a and b show the structure of carbon foams using 1.6 and 3.2 P/F molar ratio. It is seen that a macroporous carbon foam structure containing plenty of pores and thin junk area was well-developed for the 1.6 P/F molar ratio, however, the 3.2 P/F molar ratio did not yield a porous structure. It means that the P/F molar ratio is an important factor to generate the desired porous structure. Therefore, 1.6 P/F molar ratio was a suitable condition for fabricating the macroporous carbon materials aimed in this study.
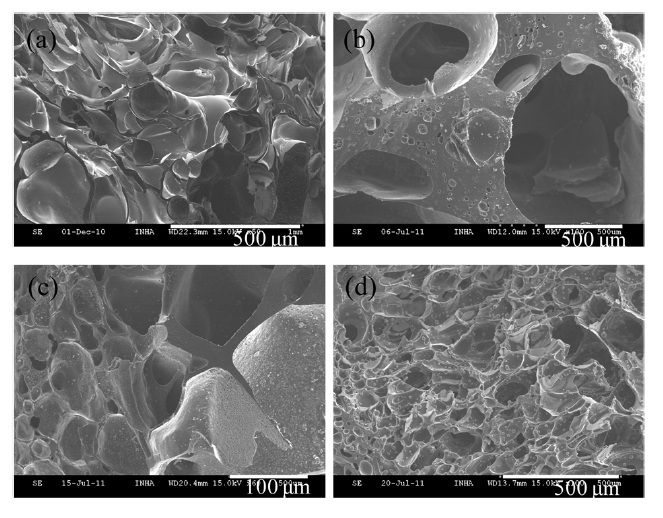
Fig. 3 shows the morphology of the resulting carbon foams prepared by 1.6 P/F molar ratio using different amounts of BMIPF6. All fabricated carbon foams have macroporous structures by the incorporation of BMIPF6 after carbonization. Fig. 3b-d reveal that junk areas and pore sizes are decreased with
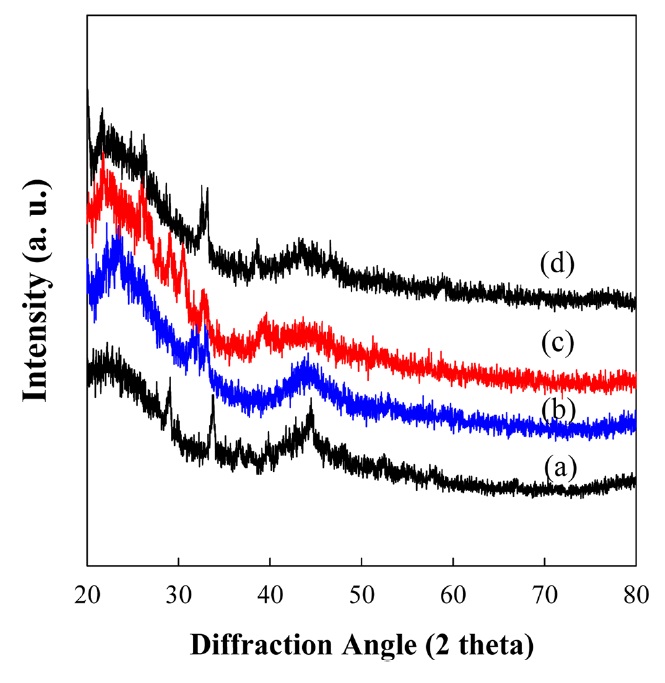
the increase in the amount of BMIPF6 in carbon foams. To the best of our knowledge, this is the first report suggesting that ionic liquids can be used as a liquid type pore generator in the fabrication of carbon foams. Fig. 4 represents X-ray diffraction (XRD) patterns of the carbon foams derived from PF resin and BMIPF6. Two broad peaks of the carbon foams could be seen at 2θ = 23 and 43℃ originating from the (002) plane of porous carbons. The (002) plane is a diffusive type and the corresponding interplaner d-spacing is 4.09 ? which is far higher than that of graphite. According to XRD patterns, it can be deduced that the resulting carbon foams were partially crystalline, but had more amorphous portion than graphite.
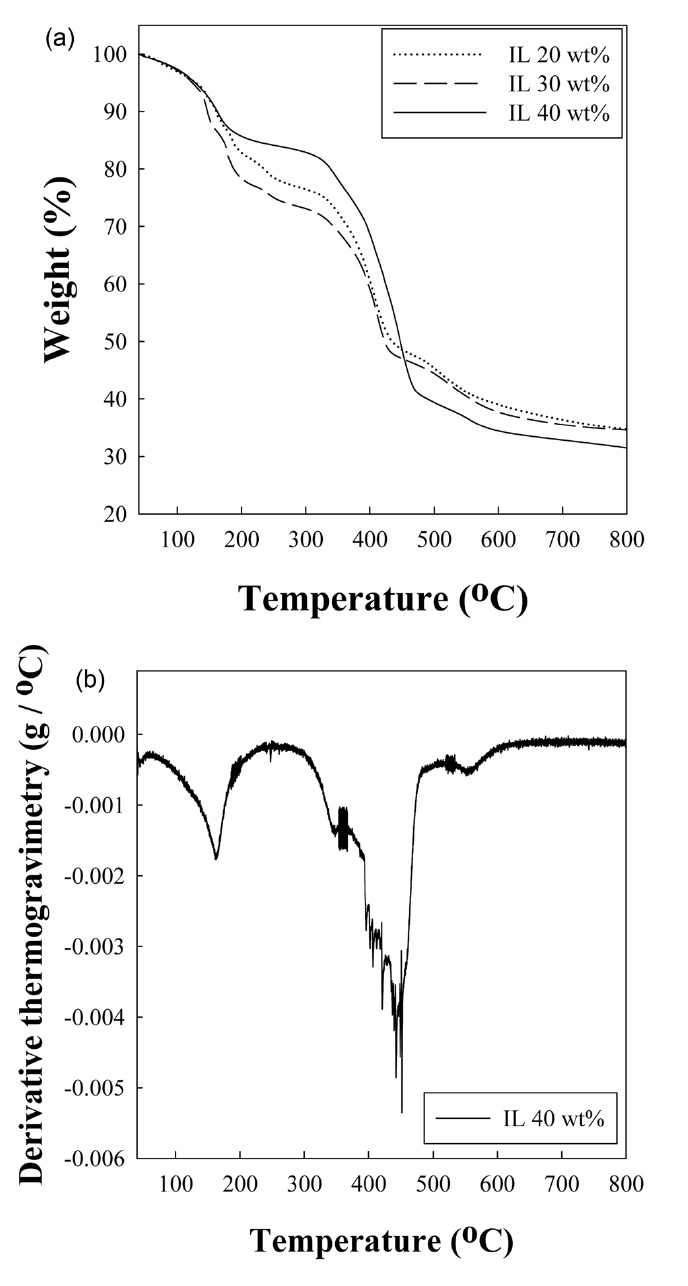
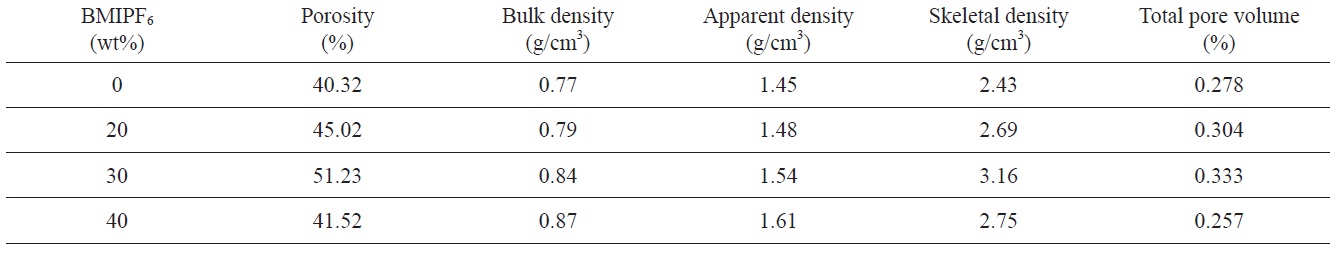
Fig. 5 shows the weight loss of cured PF body containing different amounts of ionic liquid upon heating. As shown in Fig. 5a, 68, 66.5% weight loss of cured resole resins is caused by the removal of unreacted materials, BMIPF6, and by-products in the cured resin [9]. Fig. 5b indicates the derivative plot of weight loss of cured body containing 40 wt% BMIPF6. The specific reaction temperature ranges were observed around 340, 450, and 530℃ , where further cross-linking and polycondensation, and carbonization reaction mainly occurred, respectively.
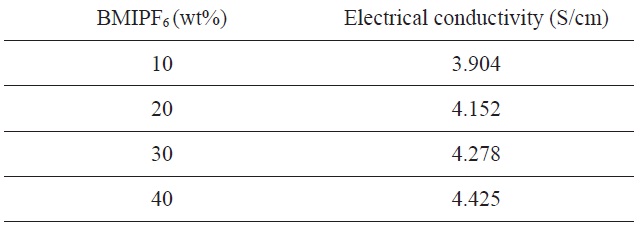
Table 1 summarizes the density and structure of pores of carbon foams prepared using different amounts of BMIPF6 as a pore generator. The porosity and total volume of carbon foams are on the order of 40, 20 and 30 wt% BMIPF6, meanwhile bulk density is in the order of 20, 30, and 40 wt%. The porosity and apparent density
[Table 1.] Properties of the carbon foams prepared with PF resin and BMIPF6
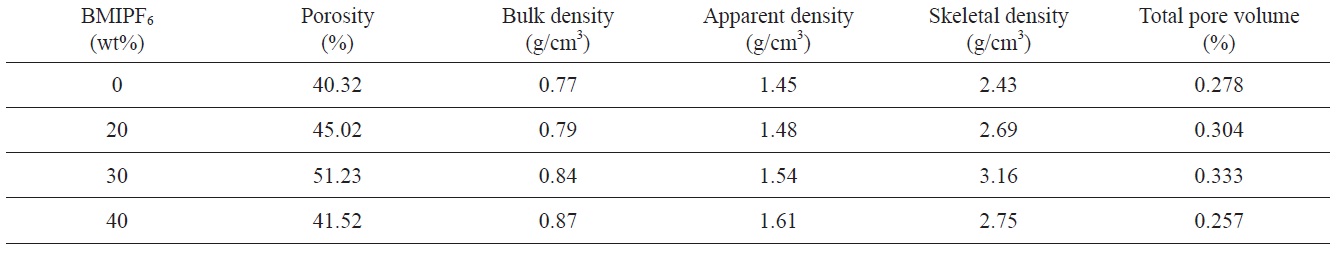
Properties of the carbon foams prepared with PF resin and BMIPF6
[Table 2.] Electrical conductivity of the carbon foams prepared by PF with BMIPF6
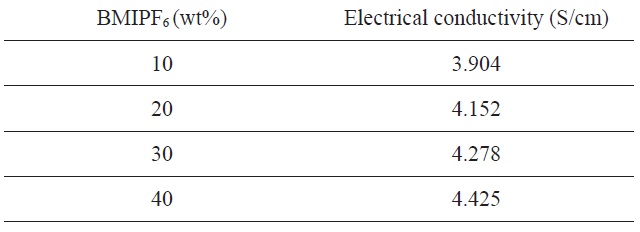
Electrical conductivity of the carbon foams prepared by PF with BMIPF6
increase from 45.02% and 1.48 g/cm3 to 51.23% and 1.54 g/cm3, respectively. But the carbon foam prepared by 40 wt% BMIPF6 has 41.52% and 1.61 g/cm3 of porosity and apparent density, respectively. During the pyrolysis, the condensation reaction released water in cured resole resin foam [10]. It is thought that the carbon foam structure is also formed by interconnection between PF and BMIPF6 during hydrothermal treatment. BMIPF6 dissolves in ethanol and water. It is assumed that BMIPF6 has a clustering behavior and water molecules are released upon heating the PF cured body, which causes the change in porosity and total pore volume. But, the carbon foam prepared by 40 wt% BMIPF6 has low porosity and total volume since the saturation of BMIPF6 disturbed its dispersion in PF resin.
Table 2 lists the electrical conductivity of carbon foams prepared using different amounts of BMIPF6. Interestingly, the electrical conductivity linearly increased from 3.904 to 4.425 S/cm with the increased amount of BMIPF6. The reason for the enhanced electrical conductivity can be found from the bulk density. Also, the ionically conductive BMIPF6 fragment may improve the electrical conductivity even after carbonization. Finally, we found that ionic liquids can be used to prepare macroporous carbon foams and the electrical conductivity could be improved by the incorporation of ionic liquids.
This paper presents a novel preparation method of macroporus carbon material using phenol/formaldehyde with an ionic liquid, BMIPF6 as a liquid type pore generator. In order to prepare macroporous carbon foam, the molar ratio of P/F was decided to be 1.6. By addition of BMIPF6 , a well-developed macroporous structure was obtained in the range of 10-40 wt% of BMIPF6. The porous structure was developed by the phase separation between PF resin and BMIPF6 during polycondensation reaction. XRD analysis reveals that amorphous-rich carbon materials with a small crystalline portion were successfully fabricated. It was seen that various aspects of the porous carbon such as porosity, bulk density, apparent density, total volume, and morphology were controllable by changing the amount of BMIPF6. Up to the 30 wt% BMIPF6, the carbon foams had higher porosity and total pore volume. Interestingly, the electrical conductivity of the carbon foams was progressively enhanced with the amount of BMIPF6 due to greater bulk density and the residual BMIPF6 fragment after carbonization.