Chalcanthite derived from the old Latin name
formed ceruloplasmin. Copper is transported by albumin and transcuprein, a high-affinity copper carrier in the plasma involved in the initial distribution of copper entering blood. Studies have discovered that transcuprein is a macroglobulin regulated by copper and iron availability [3, 7]. The main ingredient of chalcanthite is CuSO4, which exists as a series of compounds that differ in their degrees of hydration [1]. The anhydrous form occurs as a rare mineral known as chalcocya-nite. The hydrated copper sulfate occurs in nature as chalcanth-ite (CuSO4? 5H2O), and two of more rare ones: bonattite (CuSO4 ? 3H2O) and boothite (CuSO4? 7H2O) [1]. Macrophages are known to play an important role in immune reactions, allergies, and inflammation. During inflammation response, macrophag-es release many different kinds of cytokines, such as interleuk-in-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF), initiating and maintaining specific immune responses as part of the immune/inflammatory cascade [8]. Lipopolysacch-aride (LPS) is a major outer membrane component of gram-negative bacteria and is frequently used as an inflammatory model due to its ability to activate macrophages. LPS stimulates immune responses by interacting with the membrane receptor CD14 to induce cytokine release through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation [7-11]. Therefore, the modulation of macrophage-mediated inflammatory responses is important in order to formulate a new therapeutic approach against these inflammatory diseases. Minerals have been traditionally used as part of oriental herbal medicine treatment, especially for immune-related diseases, in Korea, China, and other Asian countries. According to oriental medicine, chalcanthite has sour, acrid, cold and toxic properti-es, and is affiliated with the liver meridian. Its main function is to eliminate toxins. Internally, it functions as a purgative and helps the body get rid of excess phlegm and toxins. Chalcanthite can also be applied externally for skin abscesses, inflammation, and the removal of necrotic tissue [12]. The traditionally used dose of chalcanthite is between 0.3 to 0.6 grams of powdered mine-ral. Larger amounts can be used if chalcanthite is being applied externally [12]. However, chalcanthite is poisonous and should be used with extreme caution. To date, due to safety issues con-cerning their cytotoxical mechanisms, mineral medicines have rarely been used. Previously, we reported that egg white combined with chalcanthite (IS4) inhibited the growth of NCI-H460 human lung cancer cells by inducing apoptotic cell death via caspase-3 activation. Mixing egg white with mineral drugs is a method to enhance pharmaceutical properties and to reduce toxicity [13]. In this study, the anti-inflammatory effects of IS4 on LPS-stimulated cytokines in mouse RAW 264.7 macrophage-like cells were investigated.
Egg white combined with chalcanthite (IS4) was used as the main experimental material. The eggs were from Hamyang, Korea, and the chalcanthite was obtained from Manila, The Phil-ippines. The chalcanthite was heated and dehydrated. During the heating process, chalcanthite uniformly received heat while being carefully watched for color change every 3 to 5 hours until it turned fully grey. The heating duration varied with the amount and the quality of the mineral used and was in the range of 10 to 24 hours. Once the chalcanthite had been dehydrated, it was cooled until the remaining heat was completely released. The moisture content of the chalcanthite ranged from 0% to 5%. After it had been cooled, the chalcanthite was finely pulverized. The pulverized chalcanthite should be stored in a concealed airtight container in a dry place to prevent any moisture from entering. The prepared chalcanthite powder was rapidly mixed with egg whites in a ratio of 30:13. The egg whites had to be ho-mogeneously mixed with a wooden spatula in a ceramic vessel so that the utensils would not interfere with or cause any chem-ical reactions during the mixing process. After the chalcanthite and egg whites had been sufficiently mixed, the mixture was cooled until the reaction heat was completely released. One-hundred mg of the attained substance was then dissolved in 1 ml of distilled water and was centrifuged at 6,000 rpm for 10 minutes. The upper level was filtered in 0.8㎛ pore sizes, and the filtered substance diluted in distilled water was used for treatment. The treatment doses of IS4 (12.5, 25, 50 ㎍/ml) were based on our previous IS4 [13].
The RAW 264.7 macrophage-like cell lines obtained from ATCC® were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and penicillin-streptomycin at 37 °C under 5% CO2.
The fetal bovine serum (FBS) was purchased from HyClone(Logan, UT) and high-glucose DMEM, L-glutamine, penicillin, and streptomycin were purchased from GIBCO BRL (Life Technologies, Grand Island, NY). Purified LPS derived from
RAW 264.7 cells were seeded into 24 well plates at 1 x 105 cells/well and were cultured in DMEM containing 10% FBS at 37°C for 24 hours. After treatment with LPS (1 ㎍/ml) for 24 hours, the concentrations of IL-1
Numerical data from each experiment were averages from triplicate samples. Experiments were repeated three times to avoid any experimental errors and to ensure the repeatability of the used methods. Data were expressed as means ± SD. Statistical differences were analyzed using the Mann?Whitney U-test in SPSS ver. 10.0 (statistical software package, Chicago, IL). P-values less than 0.05 were considered statistically significant.
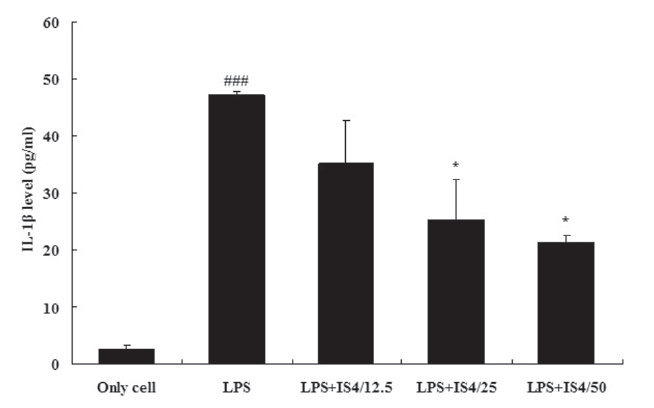
3.1. IL-1βexpression in RAW 264.7 cells
To test the effect of IS4 on LPS-induced IL-1
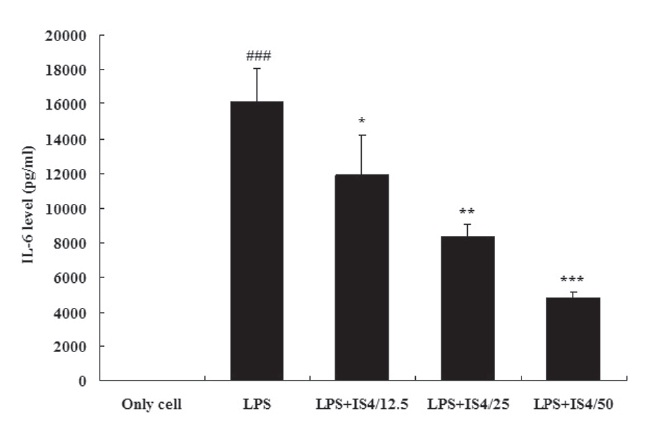
3.2. IL-6 expression in RAW 264.7 cells
To test the effect of IS4 on LPS-induced IL-6 production, we cultured RAW 264.7 cells with LPS (1 ㎍/ml) and various conce-ntrations of IS4 (12.5, 25, 50 ㎍/ml) for 24 hours. The IL-6 conce-ntration in the supernatants was determined by using an ELISA. As shown in Fig. 2, stimulation with LPS showed 99.9% increase in the expression of IL-6 in RAW 264.7 cells when compared to the untreated control (P〈 0.001). Moreover, the cells treated with IS4 inhibited IL-6 expression in a concentration-dependent manner, which showed 26% inhibition with 12.5 ㎍/ml (P〈 0.05), 48% inhibition with 25 ㎍/ml (P〈 0.01), and 70% inhibition with 50㎍/ml (P〈 0.001).
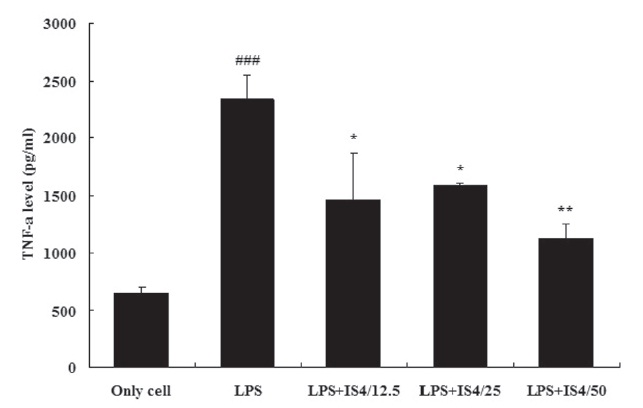
3.3. TNF-α expression in RAW 264.7 cells
To test the effect of IS4 on LPS-induced TNF-
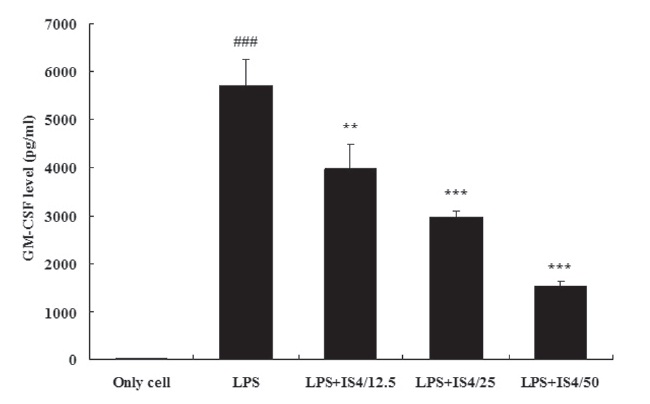
3.4. GM-CSF expression in RAW 264.7 cells
To test the effect of IS4 on LPS-induced GM-CSF production, we cultured RAW 264.7 cells with LPS and various concentrations of IS4 (12.5, 25, 50 ㎍/ml) for 24 hours. The GM-CSF concentration in the supernatants was determined by using
an ELISA. As shown in Fig. 4, stimulation with LPS (1 ㎍/ml) remarkably increased the expression of GM-CSF in RAW 264.7 cells when compared to the untreated control (P〈 0.001). Moreover, the cells treated with IS4 inhibited of GM-CSF expression in a concentration-dependent manner, which showed 30% inhibition with 12.5 ㎍/ml (P〈 0.01), 48% inhibition with 25 ㎍/ml (P〈 0.001), and 73% inhibition with 50 ㎍/ml (P〈 0.001).
The results were analyzed using Korea Basic Science Institute (KBSI)’s EAS (Expression Analysis System).
Previously, we had tested the efficacy of IS3 (heat treated chalcanthite), IS4 (egg white combined with chalcanthite), and IS5 (raw chalcanthite) on NCI-H460 human lung cancer cells. Among them, the egg-white treated IS4 most successfully inhibited the growth of cells by inducing apoptotic cell death via caspase-3 activation [13]. Other than being a quality protein source, egg whites contain many essential minerals, including potassium, magnesium, calcium, phosphorus, copper, zinc, and iron. They are also a good source of riboflavin and selenium,along with essential vitamins such as folate, B12, niacin betaine, and choline [14]. Historically, in medieval times in the West, egg whites were used for medicinal purposes, such as treating wounds, skin disorders, and mending broken bones [15]. Today, egg whites are considered to be one of the most nutrition-dense foods and are widely recommended for children, the elderly, athletes, and patients. Copper has been used in France since the 18th century to stabilize egg foams. Reactive sulfur items, such as egg whites beaten in a copper bowl, create tighter bo-nds. The bonds created are so tight that the sulfur atoms from the egg whites are prevented from reacting with any other materials [15]. Avidin is a tetrameric biotin-binding glycoprotein found in raw egg white. In chicken egg white, avidin makes up approximately 0.05% of the total protein (approximately 1.8 mg per egg) [16]. The tetrameric protein contains four identical subunits (homotetramers), each of which can bind to biotin (vitamin B7) with a high degree of affinity and specificity [16]. Research in the 1970s helped establish the avidin-biotin system as a powerful tool in biological sciences [17]. Avidin|s affinity for biotin is applied in wide-ranging biochemical assays, including western blot, ELISA, ELISPOT and pull-down assays. Avidin immobilized onto solid supports is also used as decontaminati-on medium to capture biotin-labeled proteins or nucleic acid molecules [17]. For example, lectins, proteins that bind specific sugar molecules on glycoproteins and glycolipids, are expressed at various levels on the surfaces of tumor cells. Conjugation of cytotoxic agents to glycoproteins recognized by lectins could be useful in the treatment of tumors. Avidin, a highly glyco-positively charged protein, contains terminal N-acetylglucosamine and mannose residues that bind to some lectins [18].
In a 1998 study, the ability of avidin targeting diffe-rent tumor models was tested through radioactively-labeled biotin conjugation. Avidin pre-targeting, followed by injection of radioactive biotin led to avidin-biotin conjugation
Egg white combined with chalcanthite may be considered as a possible future candidate for an anti-inflammatory agent. Furt-her studies are needed to evaluate the inhibitory mechanisms toward pro-inflammatory cytokines and should focus on
>
Appendix A. Supplementary data
An online supplementary data associated with this article can be found at doi:10.1016/j.cyto.2008.03.010.
Single and repeated oral dose toxicity tests of IS4 were performed and confirmed its safety. The results are currently under review and will be published in the near future.