Various diseases such as allergic asthma, allergic rhinitis and atopic dermatitis belong to type I allergy which is caused by immunological hypersensitivity against specific antigen such as foods, dust mites, pollen, and so on. These antigens product the antigen-specific immunoglobulin E (IgE), that binds to high affinity IgE receptor (FcεRI) on the surface of mast cells or basophils. Antigen-induced cross-linking of FcεRI initiates mast cell activation and triggers allergic inflammatory reaction, which is a cascade of events leading to degranulation and the production of cytokines via intracellular signaling pathways [1].
In this study, we investigated effects of Rehmannia Glutinosa pharmacopuncture solution (RGPS) on the degranulation, the production of cytokines, and the expression of several cytokine genes via FcεRI signaling in RBL-2H3 cells.
1. Plant Material and Preparation of Solution
RGPS purified from Roots of R. glutinosa. The preparation of ethanol extracts of
The anti-dinitrophenyl(DNP) IgE and DNP- human serum albumin(HSA) were purchased from Sigma (St. Louis, MO, USA). All of ELISA kits were obtained from BD Biosciences (Franklin Lakes, NJ, USA). anti-phospho-extracellular signal-regulated kinase (pERK), anti- extracellular signal-regulated kinase (ERK), anti-phospho-c-Jun NH2-terminal kinase (pJNK ), anti- c-Jun NH2-terminal kinase (JNK), anti-phospho p38 MAPK (pp38 MAPK), anti-p38 MAPK, anti-NF-κB (p65), and anti-β-actin were from Cell Signaling Technology. The ECL chemiluminescence system was from Amersham, and the nitrocellulose transfer membrane was from Whatman GmbH. TRIzol was obtained from Invitrogen (Carlsbad, CA, USA). All other reagents were of the highest grade commercially available.
RBL-2H3 cells were cultured at 37°C in Dulbeco's Modified Eagle's Medium (DMEM) (Hyclone, USA) containing 10% fetal bovine serum (FBS) (Hyclone, USA), 100 U/ml of penicillin, and 100 U/ml of streptomycin in an atmosphere of 5% CO2. Cells were detached with trypsin?EDTA solution. After washing, the cells were resuspended in fresh medium and used for subsequent experiments.
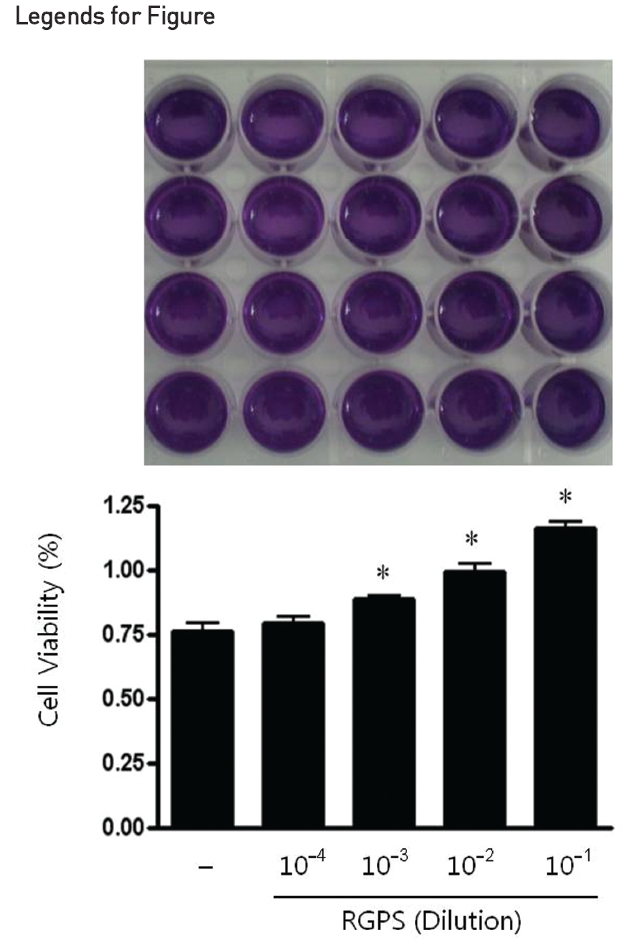
Cell viability was estimated by the MTT assay [10]. RBL-2H3 cells were incubated into 24-well plate (5×104 cells/ml) and cultured overnight. After incubation, the culture medium was replaced with the complete growth medium and then cells were treated with or without RGPS (10-4, 10-3, 10-2, and 10-1 dilution) for 1h at 37°C, 5% CO2. MTT (5 mg/ml) were added to each well and the plates were incubated at 37°C in the dark for 4 h. The supernatant of each well was vacuum-aspirated and 200 μl of dimethyl sulfoxide (DMSO) was added to each well. The plates were agitated to enhance the dissolution of the formazan formed. Cell viability was determined by a microplate reader (Spectra Max M2, Molecular Devices Co., Menlo Park, CA, USA) at 570 nm.
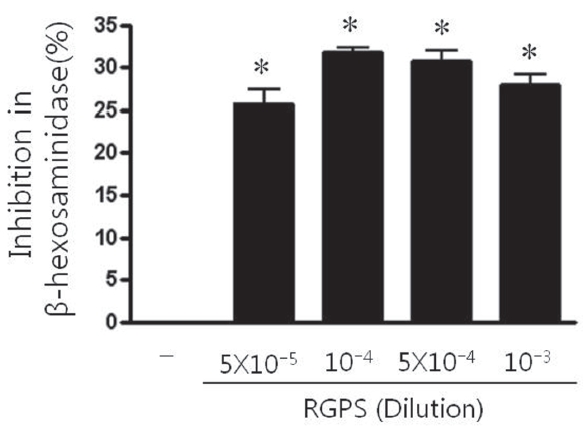
Degranulation was determined by measuring release of the granule marker, β-hexosaminidase. RBL-2H3 cells (5×105 cells/ml) were seeded into 48-well plate and sensitized with anti-DNP IgE (0.5 μg/ml) overnight. The IgE-sensitized RBL-2H3 cells were washed twice with Siraganian buffer I (119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 25 mM PIPES, 40 mM NaOH, pH 7.2), they were replaced with Siraganian buffer II (119 mM NaCl, 5 mM KCl, 5.6 mM glucose, 0.4 mM MgCl2, 1 mM CaCl2, 25 mM PIPES, 40 mM NaOH, 0.1% BSA, pH 7.2). Cells were pretreated with or without RGPS (5×10-5, 10-4, 5×10-4, and 10-3 dilution) that dissolved in Siraganian buffer II containing 1% ethanol for 1 h and then stimulated with 10 μg/ml DNP-HSA for 1 h at 37°C, 5% CO2. To stop reaction, the plate was put on ice for 10 min. Then 20 μl of supernatants were transferred to 96-well plate and incubated with 80 μl of substrate solution (1 mM p-nitrophenyl-N-acetyl-β-D-glucosaminide in citrate 0.05 M, pH 4.5) for 30 min at 37°C. The reaction was terminated by adding 100 μl of stop solution. The absorbance was measured with a microplate reader (Molecular Devices) at 405 nm. The percent inhibition of β-hexosaminidase release from RBL-2H3 cells by the test sample was calculated using the following equation:
Inhibition(%) =(1-T/C)×100
C: Cell (+), DNP-HSA (+), test sample (?);
T: Cell (+), DNP-HSA (+), test sample (+)
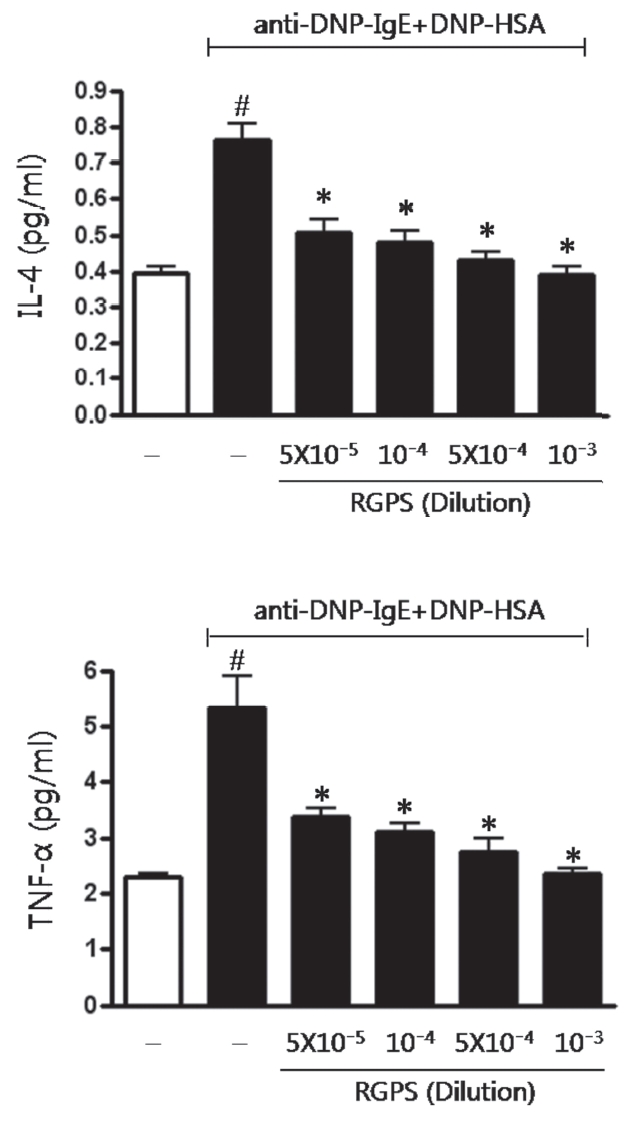
RBL-2H3 cells (5×105 cells/ml) were seeded into 24-well plate and sensitized with anti-DNP IgE (0.5 μg/ml) overnight. The IgE-sensitized RBL-2H3 cells were washed twice with Siraganian buffer I, they were replaced with Siraganian buffer II. Cells were pretreated with or without RGPS (5×10-5, 10-4, 5×10-4, and 10-3 dilution) that dissolved in Siraganian beffer II containing 1% ethanol for 1 h and then stimulated with 10 μg/ml DNP-HSA for 4 h at 37°C, 5% CO2. To stop reaction, the plate was put on ice for 10 min and then supernatants were transferred to e-tubes. Supernatants were centrifuged at 5,000 rpm for 10 min and transferred to new e-tubes. The level of IL-4 and TNF-α concentration was determined by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions.
7. RNA preparation and mRNA analysis by RT-PCR
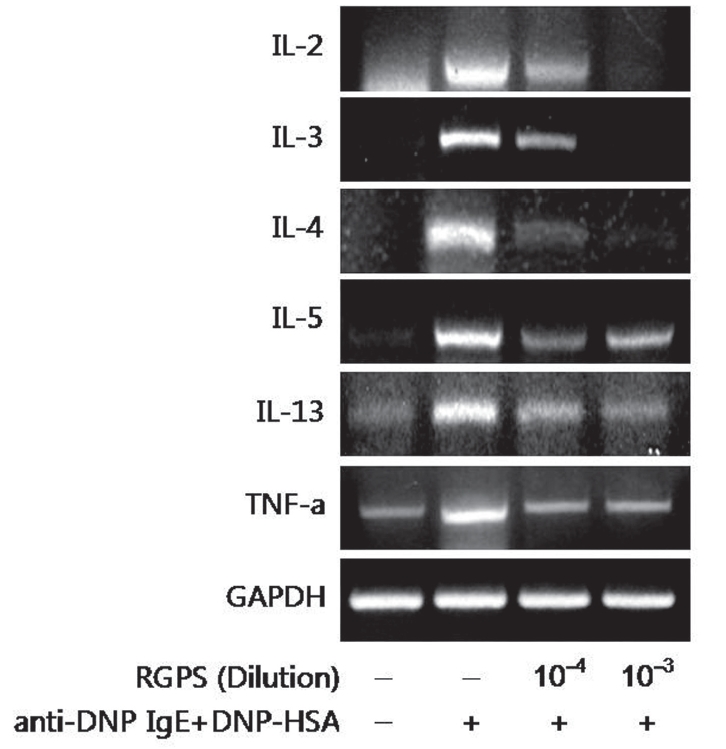
RBL-2H3 cells (1×106 cells/ml) were seeded into 6-well plate and sensitized with anti DNP-IgE (0.5 μg/ml) overnight. The IgE-sensitized RBL-2H3 cells were washed twice with PBS and replaced with the complete growth medium. Cells were pretreated with or without RGPS (10-4 and 10-3 dilution) in a complete growth medium containing 1% ethanol for 1 h at 37°C and then stimulated with DNP-HSA (10 μg/ml) for 4 h at 37°C, 5% CO2. The cells were then chilled with ice to terminate the stimulation. Thereafter, the cells were washed twice with ice-cold PBS and then total RNA was extracted from the cells by using TRIzol reagent according to the manufacturer’s instructions. The following primers were used: IL-2, 5’-CTG TGT TGC ACT GAC GCT TGT C-3’ and 5’-CTG AGT CAT TGT TGA GAT GAT GC-3’ (444 bp); IL-3, 5’-GTA TGC TGC TCC CGC TCC TGA TG-3’ and 5’-CAT TCC ACG GTC ATA GGG CGA AAG-3’ (473 bp); IL-4, 5’-ACC TTG CTG TCA CCC TGT TC-3’ and 5’-TTG TGA GCG TGG ACT CAT TC-3’ (351 bp); IL-5, 5’-CTC TGT TGA CGA GCA ATG AG-3’ and 5’-CTC TTG CAG GTA ATC CAG GA-3’(239 bp); IL-13, 5’-GCT CTC GCT TGC CTT GGT GGT C-3’ and 5’-CAT CCG AGG CCT TTT GGT TAC AG-3’ (276 bp); TNF-α, 5’-CAA GGA GGA GAA GTT CCC AA-3’ and 5’-CGG ACT CCG TGA TGT CTA AG-3’ (501 bp). The denaturation, annealing, extension, and cycle conditions were as follows: : IL-2: 94°C for 30 seconds, 60°C for 45 seconds, 72°C for 60 seconds and 34 cycles; IL-3: 94°C for 30 seconds, 60°C for 45 seconds, 72°C for 60 seconds and 24 cycles; IL-4: 94°C for 30 seconds, 58°C for 45 seconds, 72°C for 60 seconds and 30 cycles; IL-5: 94°C for 30 seconds, 58°C for 45 seconds, 72°C for 60 seconds and 35 cycles; IL-13: 94°C for 30 seconds, 60°C for 45 seconds, 72°C for 60 seconds and 25 cycles; TNF-α: 94°C for 30 seconds, 60°C for 45 seconds, 72°C for 60 seconds and 25 cycles. The PCR reaction was performed with a GeneAmp PCR System 9700 (Applied Biosystems). The PCR products were electrophoresed in 2% (w/v) agarose gels and stained with Ethidium Bromide (EtBr) (Amresco). The detection and densitometric analysis of bands were performed with a Scion Image (Scion Corporation). The sizes of bands were confirmed with reference to molecular size markers (100 bp DNA Ladder Marker, Invitrogen). The value of each cytokine mRNA was normalized to the amount of GAPDH mRNA, which was utilized as a housekeeping gene for each experimental condition
RBL-2H3 cells (1×106 cells/ml) were seeded into 100 mm dish and sensitized with anti DNP-IgE (0.5 μg/ml) overnight. The IgE-sensitized RBL-2H3 cells were washed twice with PBS and replaced with the complete growth medium. Cells were pretreated with or without RGPS (10-4 and 10-3 dilution) in a complete growth medium and 1% ethanol for 1 h at 37°C and then stimulated with DNP-HSA (10 μg/ml) for 15 min (for MAPK) and 1 h (for NF-κB) at 37°C, 5% CO2. The cells were then chilled with ice to terminate the stimulation. Thereafter, the cells were washed twice with ice-cold PBS and lysed in 0.5 ml with an ice-cold lysis buffer (20 mmol/L HEPES [pH 7.9], 0.4 mmol/L NaCl, 1% Nonidet P-40, 10% glycerol, 5 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L DTT, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.5mmol/L PMSF). The lysates were kept on ice for 30 min, followed by centrifugation at 15,000g for 15 min at 4°C. The proteins were separated with SDS-PAGE and were then transferred to nitrocellulose transfer membranes (Whatman, GmbH). Subsequent to blocking in a TBS-T buffer (10 mmol/L Tris-HCl [pH 7.5], 150 mmol/L NaCl, and 0.05% Tween 20) containing 5% skimmed milk powder, the membrane was incubated with individual antibodies. The primary antibodies were diluted 1:1000-fold unless otherwise noted and were incubated at 4°C overnight. The membranes were washed 3 times for 5 min each with a TBS-T buffer. The immunoreactive proteins were incubated with the use of horseradish peroxidase?coupled secondary antibodies diluted 1:2000-fold for 1 h at room temperature, subsequently washed 3 times (10 min each wash) with a TBS-T buffer, and developed with enhanced chemoluminescence, according to the manufacturer’s protocols (Amersham Biosciences, Piscataway, NJ).
Data are presented as means ± SD. Statistical analysis of the data was evaluated by oneway analysis of variance (ANOVA) followed by the least significant difference. Differences among groups were analyzed using Dunnett’s test and those at p < 0.05 were accepted as significant.
1. Effect of RGPS on Cell Viability of RBL-2H3 Cells
We used the MTT assay to assess the cell viability of RGPS on RBL-2H3 cells which were treated for 1 h at a dilution of 10-4, 10-3, 10-2, and 10-1. The results show that RGPS in the range of 10-4 to 10-1 dilution did not cause any cytotoxicity after 1 h (Fig.1 ).
2. Effect of RGPS on the β-Hexosaminidase Release
The β -hexosaminidase is the granule marker implicating in degranulation of mast cell. Thus, we investigated whether RGPS suppressed the release of β ?hexosaminidase in antigen-stimulated RBL-2H3 cells. The β -hexosaminidase release was induced by the antigen DNP-HSA in IgE-sensitized RBL-2H3 cells. The β-hexosaminidase release inhibition effect of RGPS (5×10-5, 10-4, 5×10-4, and 10-3 dilution) on RBL-2H3 cells is shown in Fig. .2 RGPS significantly inhibited antigen-induced mast cell degranulation.
3. Effects of RGPS on the level of IL-4 and TNF-α
Multiple cytokines produced in mast cells play a pivotal role in allergic inflammation[11]. Accordingly, we examined whether RGPS suppressed the secretion of cytokines IL-4 and TNF-α in antigen-stimulated RBL-2H3 cells. Compared to the basal level of IL-4 and TNF-α, the level of IL-4 and TNF-α in IgE-sensitized RBL-2H3 cells markedly increased after antigen stimulation (Fig.3 ). The level of IL-4 and TNF-α on RBL-2H3 cells was inhibited by RGPS (5×10-5, 10-4, 5×10-4, and 10-3 dilution) in a dose-dependent manner (Fig.3 ).
4. Effects of RGPS on the mRNA expression of cytokines
Effects of RGPS on the mRNA expression of cytokines We examined whether RGPS suppressed the mRNA expression of various cytokines; IL-2, IL-3, IL-4, IL-5, IL-13 and TNF-α in antigen-stimulated RBL-2H3 cells. The effect of 10-4 and 10-3 diluted RGPS on the induction of these cytokines at 4h after antigen stimulation was investigated. RGPS significantly suppressed induction of these cytokines expression in a dose-dependent manner (Fig.4 ).
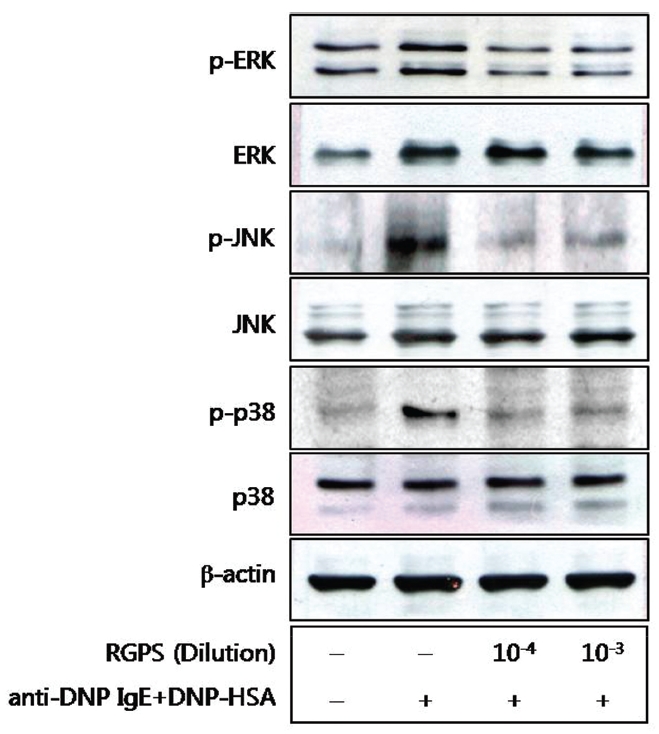
5. Effects of RGPS on the phospholyation of ERK, JNK, and p38
We also examined the effects of RGPS on the MAPKs[12],[13] because of their role in the production of cytokines. The activating phosphorylation of the ERK, JNK, and p38 were also suppressed by RGPS (Fig.5 ).
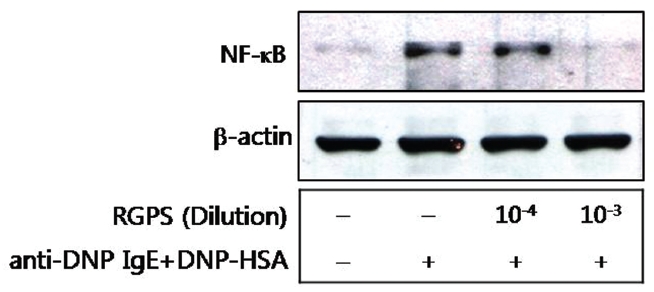
6. Effects of RGPS on the translocation of NF-κB
We also examined effects of RGPS on the translocation of NF-κB from cytoplasm to nuclear. The translocation of NF-κB was significantly inhibited by the 10-3 diluted RGPS than 10-4 diluted RGPS in particular (Fig.6 ).
In this study, we evaluated the effects of RGPS on IgE mediated allergic reaction in RBL-2H3 cells and elucidated molecular mechanisms underlying the RGPS effects. We first estimated the cytotoxicity of RGPS in the range of 10-4 to 10-1 dilution prior to investigate anti-allergic effects of RGPS. Our results show that RGPS did not cause any cytotoxicity and increased cell viabilities in the range of 10-3 to 10-1 dilution (Fig.1 ). The activated mast cells immediately release preformed chemical mediators such as histamine, β-hexosaminidase and serotonin in the early stage of a type I allergic reaction.
We demonstrated whether RGPS suppressed the release of β-hexosaminidase caused by aggregation of FcεRI in RBL-2H3. Our results show that RGPS significantly suppressed the release of β-hexosaminidase, implicating in degranulation of mast cells in the early stage (Fig.2 ). Mast cells are a rich source of multifunctional cytokines.[14] These cytokines produced from the activated mast cells are associated with the development of allergic inflammation in the late stage of a type I allergic reaction [1, 11, 15-17]. Thus we investigated whether RGPS suppresses the up-regulation of expression of some cytokines caused by aggregation of FcεRI in RBL-2H3. Our results show that RGPS effectively suppressed the production of IL-4 and TNF-α (Fig.3 ) and the mRNA expression of IL-2, IL-3, IL- 4, IL-5, IL-13, and TNF-α (Fig.4 ). We subsequently evaluated the molecular mechanisms related to cytokine secretion, the phosphorylation of MAPKs (ERK, JNK, and p38) and NF-κB activation. MAPKs and NF-κB are an essential signal for the production of cytokines such as IL-4 and TNF-α in FcεRI mediated signal transduction [12, 18-23]. In unstimulated condition, NF-κB exists in an inactive state in the cytoplasm complexed to the inhibitory IκB molecules. Upon stimulation, NF-κB is dissociated from the IκB and translocated to the nucleus, and activate the expression of inflammatory cytokines. [24] Our results show that RGPS suppressed the phospholylation of ERK, JNK, and p38 (Fig.5 ). In addition, our results show that RGPS inhibited nuclear translocation of NF-κB (p65) (Fig.6 ).
In conclusion, this study indicates that RGPS inhibits degranulation, cytokine secretion, and FcεRI-mediated signal transduction in antigen-stimulated RBL-2H3 cells.
These results suggest that RGPS exhibits an anti-allergic potential activity for the treatment of allergic inflammatory diseases through the down-regulation of mast cell activation.
86
87
88
89