Activated carbons (ACs) were highly microporous materials with a large specific surface area and have been employed as one of the main adsorbents for desulfurization [1]. The method of removing CS2 by using activated carbon at normal temperature was considered to be economical and appropriate. Recently, the ACs are considered as an efficient and convenient materials for adsorption of CO2 pollution from atmosphere which is the chief man-made global warming gas.
CO2 adsorption onto ACs generally depends on their physical and chemical properties. Physical properties of carbons include their specific surface area, size, and porosity,whereas chemical properties are mainly determined by their surface functional groups, including carboxyl, carboxylic anhydrides and so on. Because of CO2 belongs to acidic pollutants, so many efforts have been focused the increasing alkalinity of ACs. Increase in alkalinity can be achieved through various techniques. The typical example is impregnation of ACs with a basic solution [2-5]. However, it should be noted that the introduction of additives through impregnation might give some other negative effects. As reported elsewhere, dipping of ACs with a solution of additives caused blocking of pores in the structure of ACs [2].
One of the popular ways used for preparation of ACs for adsorbing CO2 was introducing nitrogen to the carbon surfaces. According to Mangun and co-workers [6], the modification of activated carbon fibers (ACFs) with dry ammonia at several temperatures from 500 to 800℃ for different periods of time results in the formation of new nitrogen-containing groups in the fibers. Xu
Therefore, in this work, we are focused on CO2 adsorption capacity of ACs modified by the amine fictionalizations. The difference between the pristine ACs and amine functionalized ACs on CO2 adsorption is compared through Boehm’s method, nitrogen full isotherms, TGA, and XPS analyses.
2. 1. Materials and Sample Preparation
The pristine ACs were heated in N2 at 10 K/min to 573 K. The 40 wt% of amines (urea, melamine, diethylenetriamine, pentaethylenehexamine, and polyethylenimine) was dispersed in 20 ml of methanol by mechanically stirring the mixture for 15 min. The solution was then poured over 2 g of ACs previously dried at 373 K overnight and the resulting slurry was kept at 333 K for 12 h min. The sorbents were, respectively, denoted as A-U, A-M, A-DE, A-PA, and A-PE, as studied by urea, melamine, diethylenetriamine, pentaethylenehexamine, and polyethylenimine. The 2 g ACs were treated with 10 ml 3-aminopropyl-triethoxysilane (ATPS) in 100 ml of toluene at 383 K for 24 h and then were washed thoroughly with the ethanol. Finally, the ACs were dried in vacuum oven at 358 K for 12 h. The samples were designated as A-AS.
The pH and basic values were determined by Boehm’s titration method [8]. N2/77 K full isotherms were measured using an ASAP 2010 (Micromeritics). Before the measurement,the samples were degassed at 473 K for 12 h to obtain a residual pressure of less than 10-6mm Hg. The changes in chemical composition of the ACs surfaces were analyzed by XPS (ESCA210, VG Scientific Co.). The X-ray source for the measurement was nonmonochromatic Mg Ka. The working pressure maintained under 10-11mbar.
The CO2 adsorption property of ACs was characterized by thermal gravimetric analysis (TGA). About 5~10 mg of the sample was heated at 10 K/min to 373 K in N2 flow (50 ml/min) and keep for 1 h, in order to degas and dehydrate the sample. After the mass decrease stabilized to a constant mass, the gas in the system was changed to pure CO2 and the temperature was maintained at 298 K for 1h. Finally, the sample was kept at 373 K and then the flow was changed again to N2 to regenerate the sample.
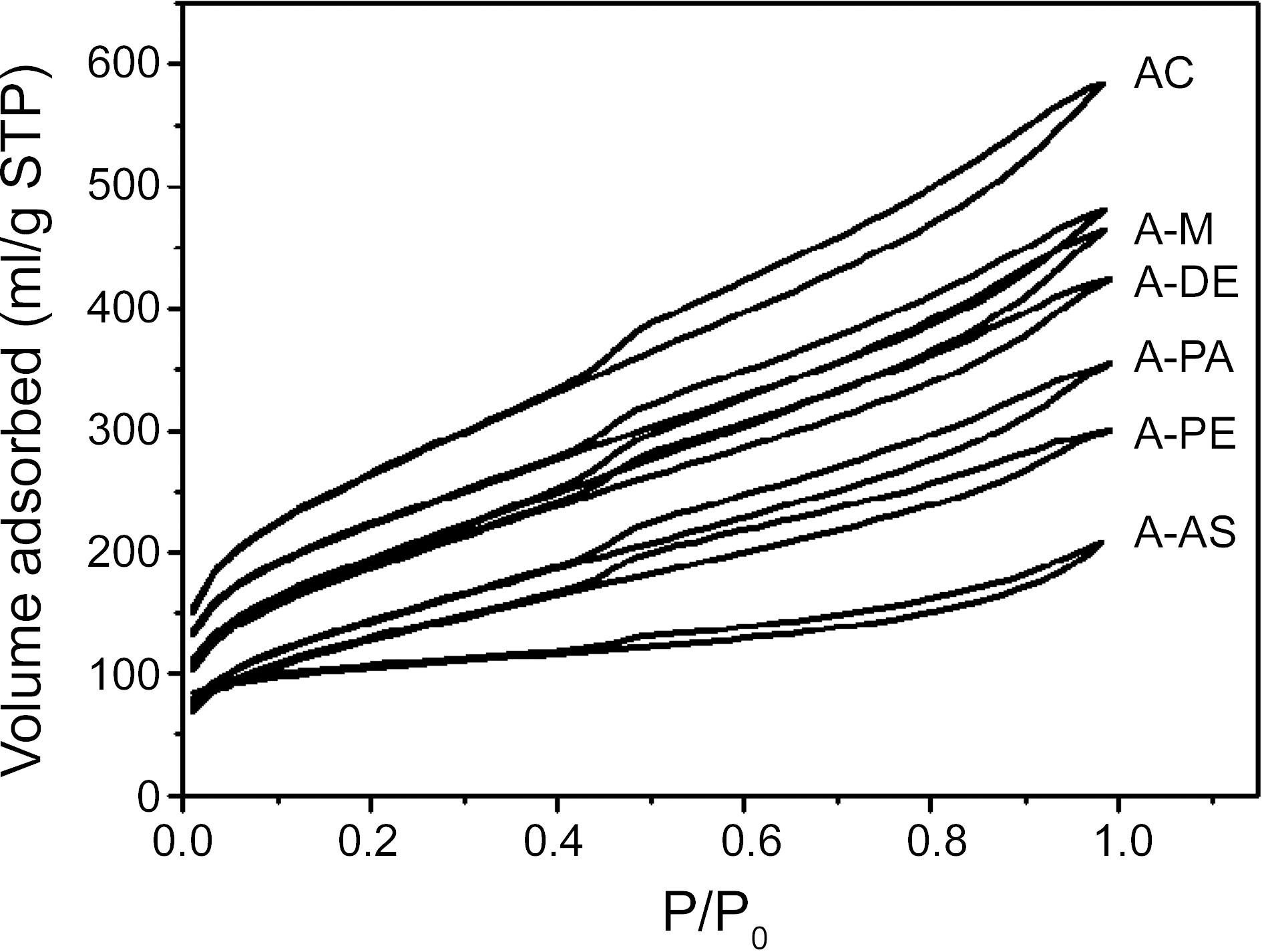
The N2 full isotherms of the pristine and amine functionalized ACs are shown in Fig. 1. The isotherms of the pristine and amine functionalized ACs show a type IV shape according to IUPAC classification [8]. The initial part of the adsorption isotherm corresponds to adsorption in the micropores. It clear shows that the surface area decrease significantly upon amine functionalization. The mesoporous adsorption step in the amine functionalized ACs is slightly less resolved than in pristine ACs due to blockage of the pores with the amines [9].
In the BET’s model [10], adsorption occurs by the formation of stacks of molecules on each surface adsorption site. The adsorption of a molecule on a vacant site is characterized by the energy, E. The difference between these two adsorption energies is called the net heat of adsorption (ΔE= E0-EL). The net heat of adsorption (ΔE) derived form BET constant (CBET) can be calculated as [11,12].
where E0 and EL are, respectively, the heat of adsorption in the first layer and the heat of liquefaction, R the gas constant, and T the Kelvin temperature.
Therefore, the difference (ΔE) between the heat of adsorption and the heat of liquefaction is, so-called, the net heat of adsorption or excess heat of adsorption in the first layer [13]. The CBET and ΔE of the amine functionalized activated carbon gradually decrease as seen in Table 1. After amine functionalization, a number of nitrogen containing functional groups exist in the crystal lattice of the surface, including electron acceptors. Therefore, the neutral N2 adsorption characteristics are greatly dependent on the surface properties as well as the pore structures of the adsorbent.
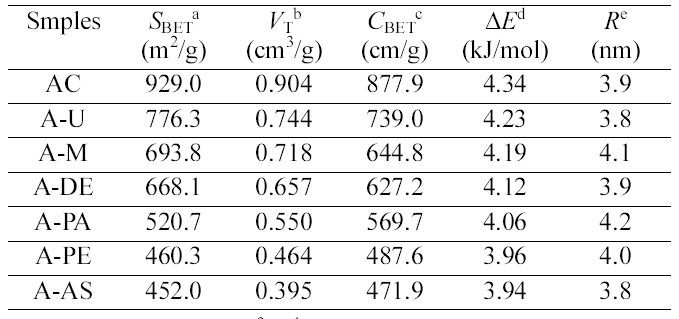
The SBET, total pore volume, and average pore diameter .of the pristine and amine functionalized ACs are collected in Table 1, which showed that amine groups change the porosity of the ACs. As expected, amine fictionalization
[Table 1.] Pore Structure Parameters for the Pristine and Amine Functionalized ACs
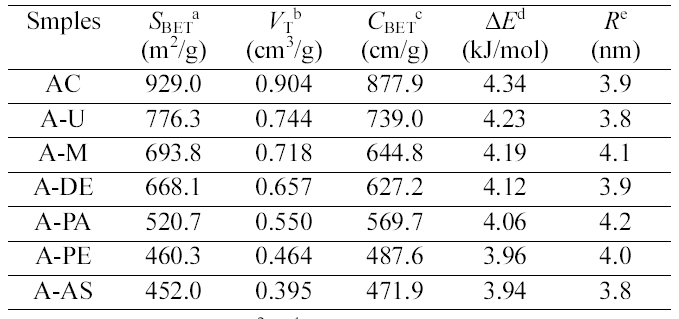
Pore Structure Parameters for the Pristine and Amine Functionalized ACs
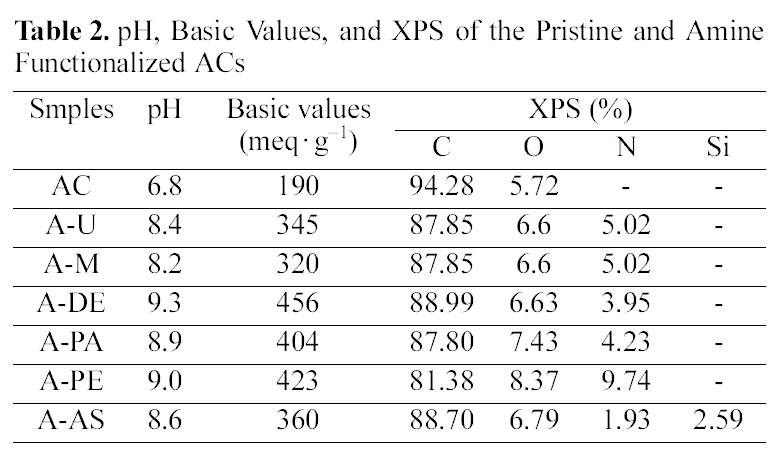
[Table 2.] pH Basic Values and XPS of the Pristine and Amine Functionalized ACs
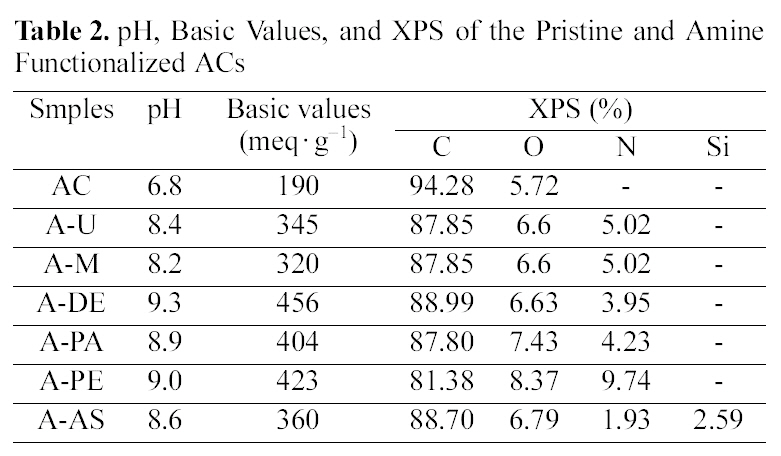
pH Basic Values and XPS of the Pristine and Amine Functionalized ACs
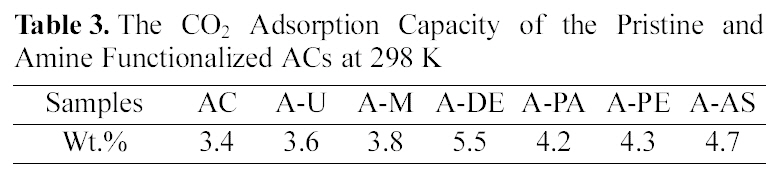
[Table 3.] The CO2 Adsorption Capacity of the Pristine and Amine Functionalized ACs at 298 K
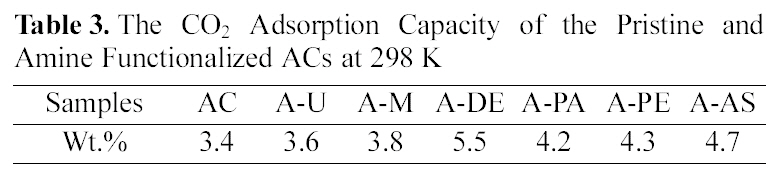
The CO2 Adsorption Capacity of the Pristine and Amine Functionalized ACs at 298 K
resulted in decrease in the surface area and total volume as a result of destruction of pore walls and micropore blocking by amine groups. However, the average pore size is increased with the amine fictionalization, which could be explained by the grafting of amines on the small pores of ACs. It also can be seen that the specific surface area and pore volume is decreased with the increasing the amine chain length, which is due to the more occupation of pore volume.
Table 2 shows pH, basic values, and XPS of the pristine and amine functionalized ACs. It can be seen that amine impregnation causes a slight shift in the pH value and basic value of the pristine ACs and amine functionalized ACs towards higher values [14]. There are different kinds and amounts of basic groups produced on the surface of ACs after different amine treatments. We can also see that the content of carbon and oxygen of the pristine ACs is 94.28%and 5.72%. After treated with different amines, the content of carbon and oxygen was decreased where the more nitrogen was incorporated into the structure. Amine fictionalizations are expected to take place at carboxylic acid sites of the ACs surfaces. Types of chemical nitrogen fixation by ACs: nitrogen bound to the carbon as aside group, such as an amide group, nitrogen chemically bound on the carbon surface through an aliphatic spacing group as a tertiary amine, nitrogen built into the edge of the graphitic planes as a lactam, or pyridine-pyrrole-like group and as a nitrile [15]. These basic groups of ACs are expected to be favourable for their application in the adsorption of an acidic gas by acid-base interaction [16].
The CO2 adsorption capacity of pristine ACs and amine functionalized ACs measured by TGA at 298 K. As shown in Table.1, the pristine ACs can adsorb 3.4 wt.% CO2 gas, this is only due to the physisorption occurring within its pore structure. The amine functionalized ACs present better performance for CO2 adsorption than the pristine ACs. The behavior of amine functionalized ACs to adsorb CO2 should be related to the number and availability of amine groups for reaction [17,18]. The order of mass of CO2 per mass of ACs is as the following order: A-DE > A-AS > A-PE > A-PA >A-M > A-U > AC. A. Arenillas
In this work, we had examined CO2 adsorbility on both pristine and amine functionalized ACs. Six different amines were successfully grafted on ACs surface through a normal impregnation method. From the TGA result, it was found that the sample A-DE showed the highest CO2 adsorption capacity at 298 K. Furthermore, the active ingredients impregnated on the ACs show significant influence on the adsorption for CO2 and its mass adsorbed on amine functionalized ACs are larger than that on the original ACs, which is due to the grafted alkaline amine groups activated with CO2 on the AC surfaces.