The wide use of heavy metal solutions in many industrial activities such as battery manufacturing and painting results in the generation of large quantities of effluent that contains high level of heavy metals. Most of them are toxic and carcinogenic. Therefore their presence in the aquatic ecosystem poses the human health risks due to their non-degradable and persistent nature. The strict regulations concerning the presence of heavy metals in the aquatic environment have been introduced in recent years [1]. Consequently, the treatment of industrial effluents is a challenging topic in the environmental field and many conventional methods are used for the removal of toxic metals from aqueous solutions such as chemical precipitation [2], liquid-liquid extraction [3], ion-exchange [4] adsorption [5,6], filtration [7,8], electrochemical treatment [9], reverse osmosis [10], membrane technologies [11], and evaporation recovery [12]. Among these methods,adsorption may be a suitable wastewater technology competitive with the other conventional technologies to remove heavy metals. Currently, research is focused on the use of bioadsorbents which are inexpensive, biodegradable, and available in large quantities to support potential demand [13].
Alginate, a natural polysaccharide extracted from brown seaweeds, is a very promising bioadsorbent. It was preferred over other materials because of its various advantages such as biodegradability, hydrophilic properties, natural origin, abundance, and presence of binding sites due to its carboxylate functions [14]. The carboxylate groups of the polymer provide the ability to form biodegradable gels in the presence of multivalent cations and more specifically with calcium ions via ionic interactions [15]. In the environmental field, alginate beads are widely used for the removal of heavy metals from wastewater [16]. In addition, alginate beads containing different components to enhance the adsorption capacity of the system are also widely investigated [17].
Carbon nanotubes (CNTs) have been widely used as adsorbents to remove inorganic and organic contaminants from wastewater in recent years. The exceptional properties of CNTs such as high mechanical strength, large specific surface area, hollow and layered structures, and high chemical and thermal stabilities, make them a good candidate as adsorbent and allow the great promising applications in environmental protection [18]. However, widespread usage of CNTs will cause the increased emissions to the water environment and result in human contact risk to CNTs [19]. Because of the poor degradability [20] and toxicity of CNTs they should be removed from the drinking water as far as possible. As it is difficult to remove CNTs from water using conventional separation methods due to their nano-sized structures, this limitation may be the bottleneck to obstruct CNTs to be widely used as adsorbents in environmental protection in the future [21].
An innovative technology that gains attention is the use of magnetic materials for the magnetic separation of pollutants from the effluents. In the environmental applications, magnetic separation can be a promising method for a novel purification technique because it produces no contaminants such as flocculants and has the capability to treat a large amount of wastewater within a short time [22]. However, the use of alginate magnetic beads remains rare in the environmental applications [23]. Nevertheless the incorporation of magnetic particles in a polymer matrix might provide several advantages such as the facile removal of beads from the effluent to reuse them after their regeneration [24].
In this study, the alginate/CNT/maghemite composite beads were prepared by w/o emulsion method in order to improve the Cu(Ⅱ) ions removal and superparamagnetic properties. The composites not only make full use of the excellent heavy metal adsorption properties of CNTs and alginate, but also prevent nano-sized CNTs from breaking off the composites to cause secondary nano-pollution to water. The composites can also be easily removed from the waste solution by the use of a magnet.
Alginic acid sodium salt (from brown algae, viscosity of 2% solution at 25℃: ~250 cps), multi-walled carbon nanotube (diameter=110-170 nm, length=5-9 ㎛, 90+%), calcium chloride (CaCl2), and iron(III) oxide (nanopowder,<50 nm) were purchased from Sigma Chemical Company. Copper standard solution was obtained from Kanto Chemical Company.
2.2. Preparation of alginate/carbon nanotube/maghemite composite beads
3 g of sodium alginate was dissolved in 97 ml distilled water to produce a viscous solution under stirring at 60℃ for 6 h. 0.6 g of CNTs was dispersed in 15 ml distilled water under sonication for 2 h. 0.25 g of iron(III) oxide was dispersed in 10ml distilled water under shaking for 1 h. 30 g calcium chloride was dissolved in 970 ml distilled water under stirring for 30 min. The CNT dispersion and iron(III) oxide dispersion were then added into the sodium alginate solution. The alginate solution containing CNTs and iron(III) oxide dripped through the injection needle into the 1000 ml solution of calcium chloride. The composite beads were formed upon contact with calcium ions. After removal from the calcium chloride bath, the beads are washed with the distilled water three times by replacing fresh distilled water every few hours to remove the excess of calcium ions and dried at -50℃ by a vacuum freeze dryer(VFD0030-5085).
2.3. Characterization of alginate/carbon nanotube/maghemite composite beads
2.3.1. SEM
The morphology of samples was investigated using JSM-7000F (Jeol, Japan) scanning electron microscopy. As a pretreatment, samples were vacuumed up to 10-3 Pa and sputtered using Pt. The SEM images were obtained at 10 keV.
2.3.2. FT-IR
Fourier transform infrared (FTIR) spectra of samples were recorded on a Nicolet 510 FTIR spectrometer, from 400 to 4000 cm-1 with a nominal resolution of 2 cm-1. The samples were pressed into tablets with potassium bromide (KBr).
2.3.3. Magnetomer
The coercive force of magnetized beads was measured by magnetic hysteresis curve in 5.00 ×103 magnetic field using 2900-02 AGIM vibrating sample magnetomer (PMC Co.).
2.3.4. BET
The surface area and pore volume of samples were measured by BET method using ASAP 2020 (Micromeritics, U.S.A.).
2.3.5. Cu(II) adsorption
The Cu(II) ion removal from the aqueous solution using alginate/CNT/maghemite composite beads was studied in a batch mode by mixing 100 ml of aqueous solution of 5 ppm Cu(II) ions with 5 g of dried composite beads. The Cu(II) ion solution containing the composite beads was shaken for 5 h at room temperature for the equilibration in adsorption. The composite beads were then easily removed from the solution by magnetic separation. The concentration of Cu(II) ions in the aqueous solution was measured using Optima 3300DV (Perkin-Elmer Instruments) Inductively Coupled Plasma Atomic Emission Spectrometer.
3.1. Morphology of alginate/carbon nanotube/maghemite composite beads
The surface morphology of alginate/CNT/maghemite composite beads was investigated by SEM as shown in Fig. 1. The CNTs were coated uniformly with alginate and dispersed in the alginate matrix. The uniformly implanted CNTs can greatly improve the surface area and the pore volume of alginate. The iron(III) oxide particles were dispersed in the alginate matrix with some agglomeration.
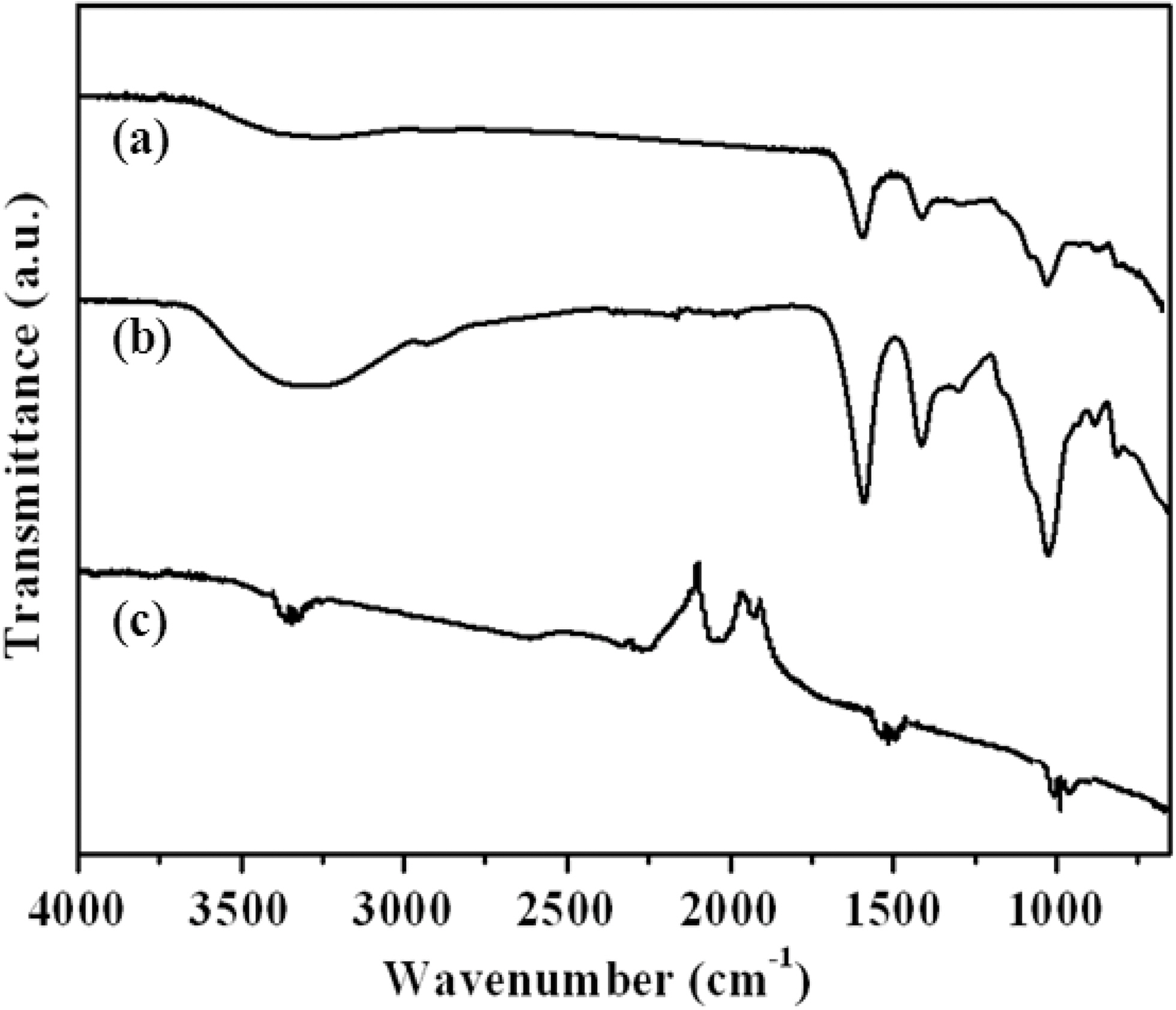
3.2. FTIR characterization of alginate/carbon nanotube/maghemite composite beads
Fig. 2 shows the FTIR spectra of alginate, CNTs, and alginate/CNT composites. The peak at 1626 cm-1 represents the -COOH stretching vibration of alginate, while the peaks at 3430 cm-1 and 1090 cm-1 are related to the -OH stretching vibration and the -C-O stretching vibrations of alginate,
respectively. These functional groups played a major role in adsorbing the heavy metal ions via ion exchange.
3.3. Magnetic property of alginate/carbon nanotube/maghemite composite beads
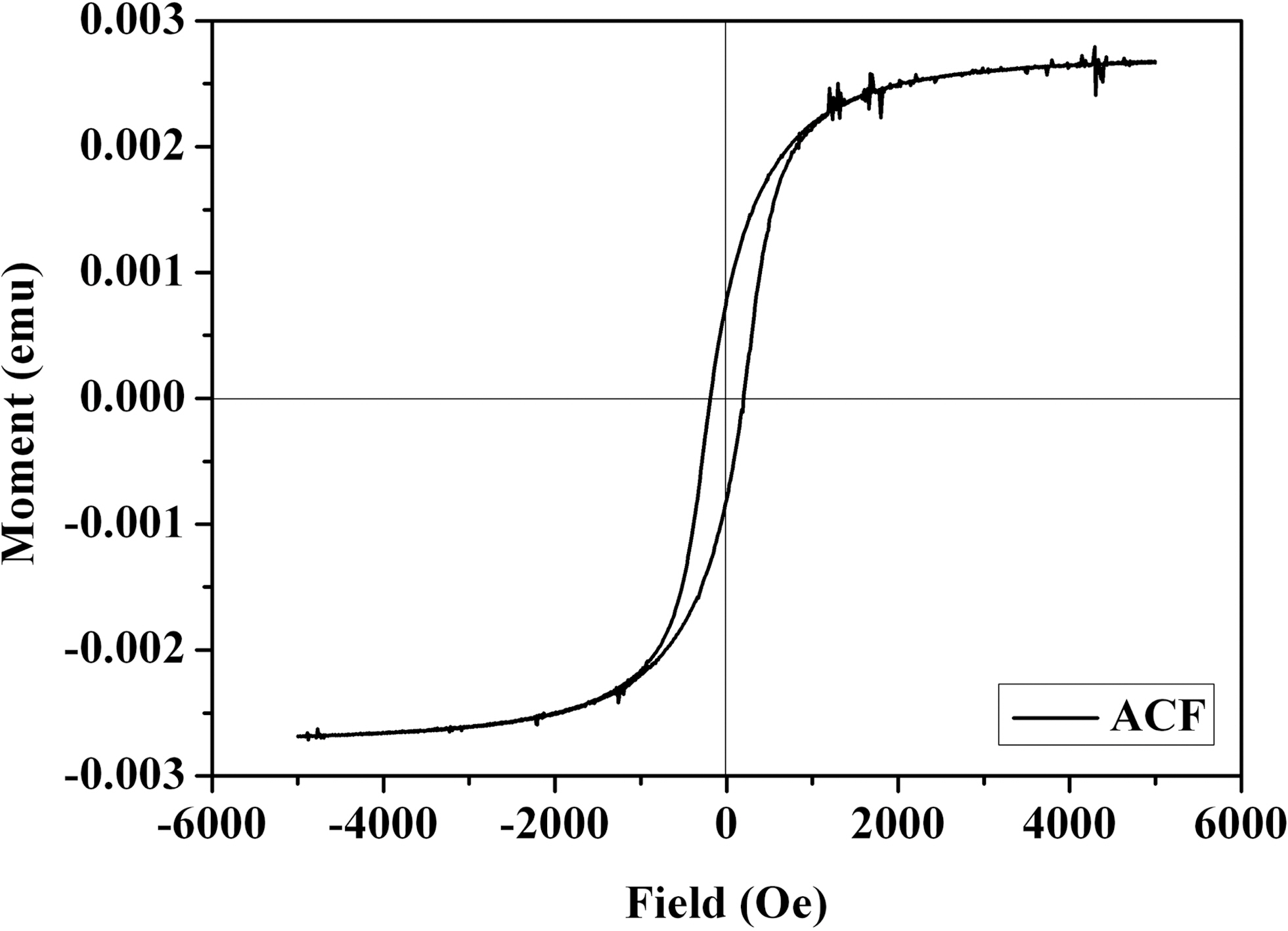
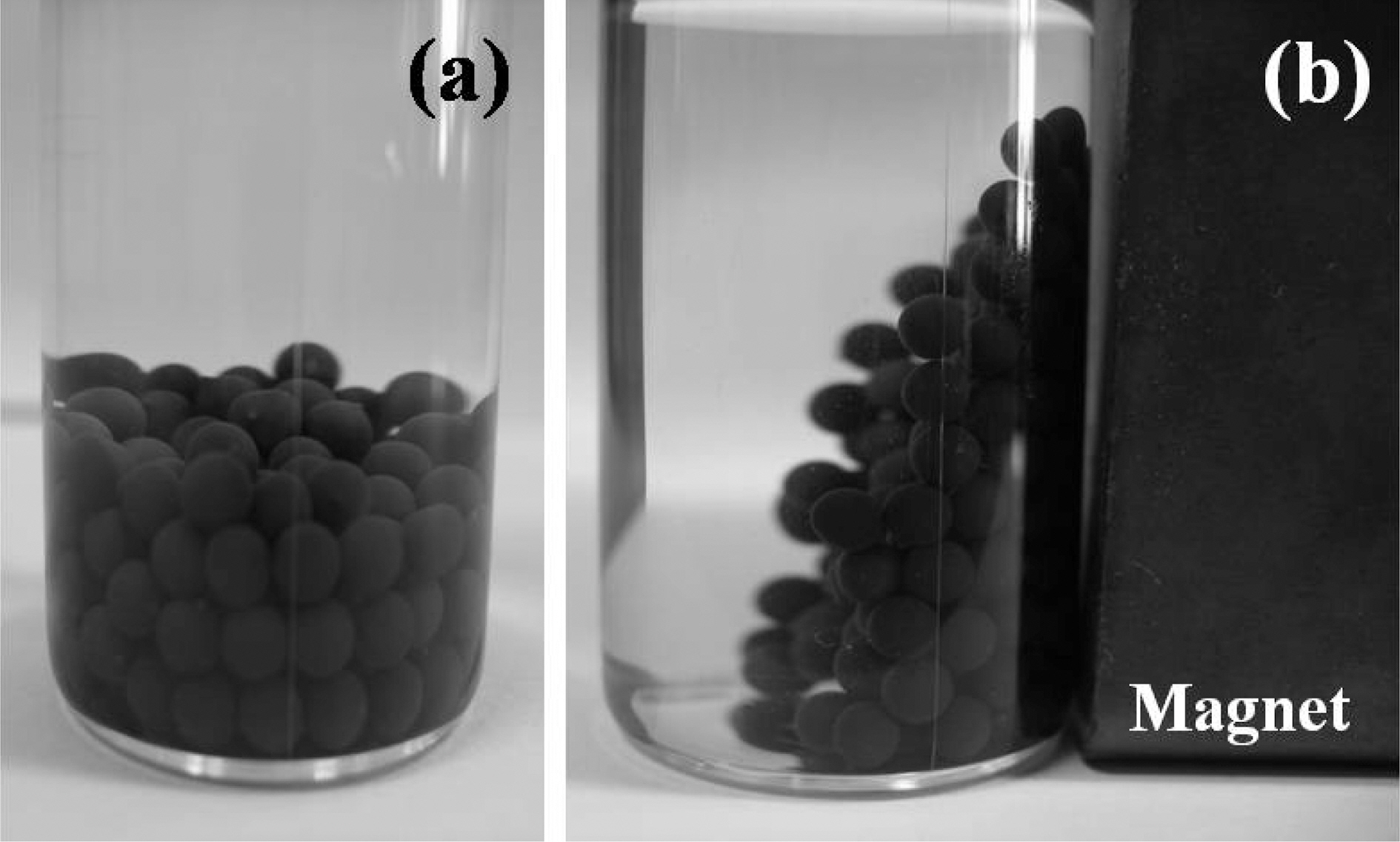
The dependence of magnetization on the applied magnetic fields for alginate/CNT/maghemite composite beads was studied with a magnetometer at room temperature and the hysteresis behavior of the composite beads is shown in Fig. 3. Ferromagnetic nature of the composite beads is clearly seen from the hysteresis curve. Based on this magnetic property, alginate/CNT/maghemite composite beads were removed conveniently from the effluent solution by the use of a magnet as shown in Fig. 4.
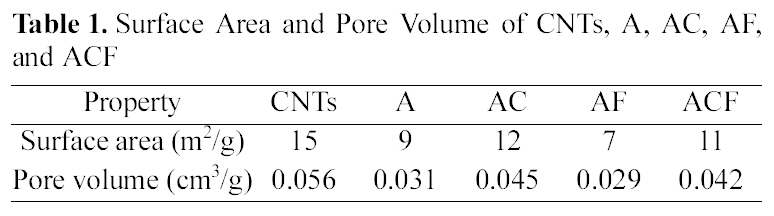
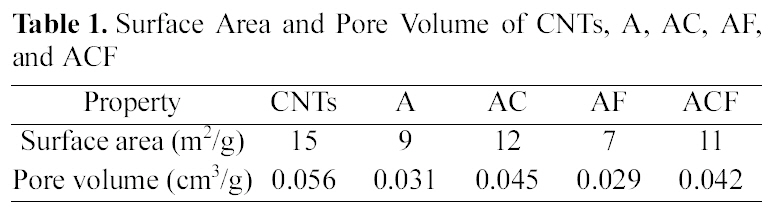
[Table 1.] Surface Area and Pore Volume of CNTs A AC AF and ACF
Surface Area and Pore Volume of CNTs A AC AF and ACF
3.4. Pore characteristics of alginate/carbon nanotube/maghemite composite beads
As shown in Table 1, the surface area and the pore volume of alginate beads were 9 m2/g and 0.031 cm3/g, respectively.Both surface area and pore volume increased noticeably to 12 m2/g and 0.045 cm3/g, respectively, by incorporating CNTs. The composite beads had a benefit of increased adsorption sites via nano-channels formed by CNTs which had the higher surface area and pore volume.
3.5. Cu(II) adsorption of alginate/carbon nanotube/maghemite composite beads
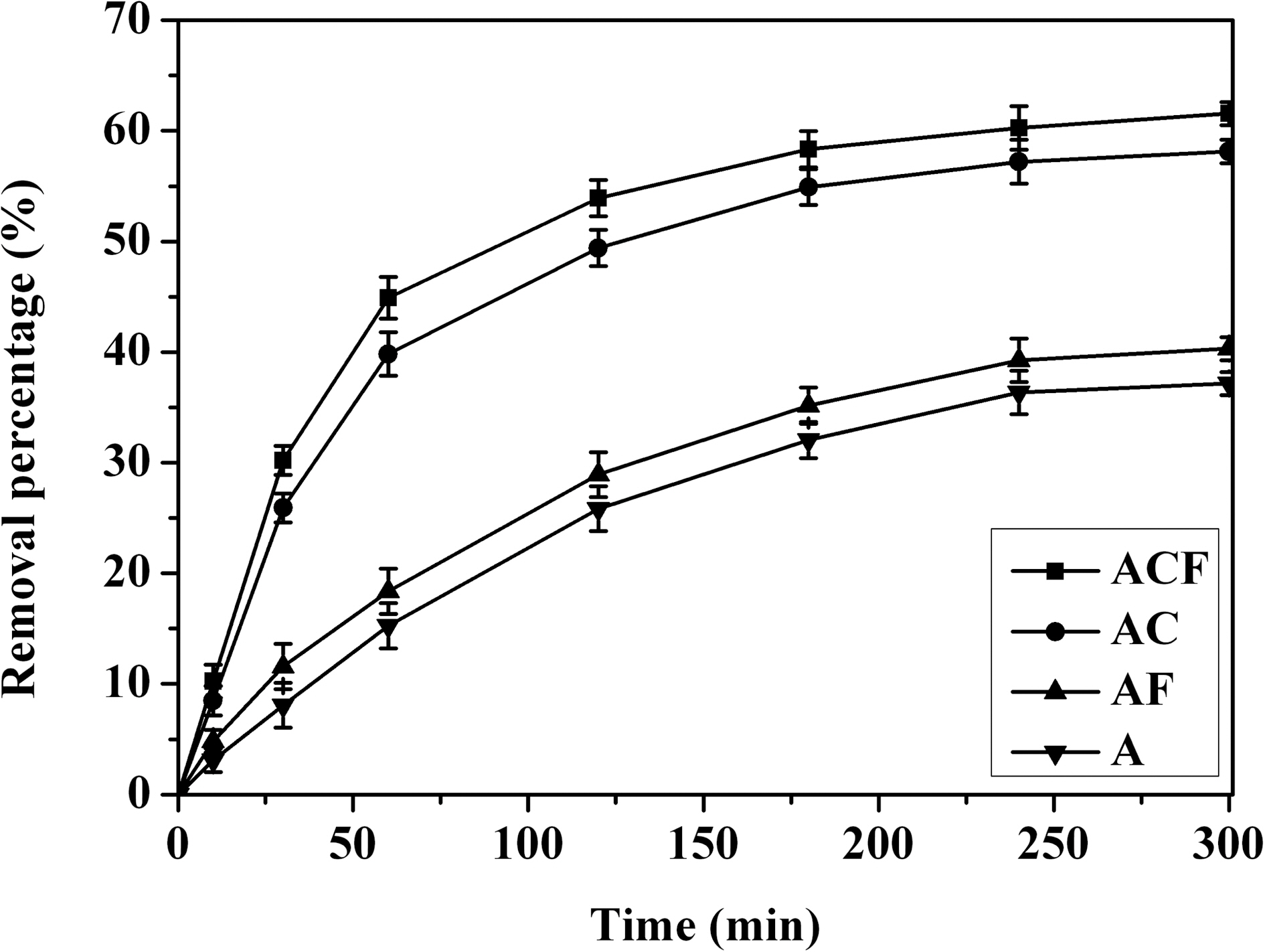
Cu(II) ion adsorption efficiency of several different adsorbents is shown in Fig. 5. The Cu(Ⅱ) ion removal efficiency stayed around 40% for the alginate beads. However, Cu(Ⅱ) ion removal efficiency improved greatly over 60% using alginate/CNT composite beads. By combining CNTs with alginate, more adsorption sites offered by the functional groups on CNTs were available additionally for the adsorption of Cu(Ⅱ) ions. This phenomenon was attributed to the synergistic effect of surface complexation and ion exchange [14,25]. The alginate/CNT composite beads could remove heavy metal ions through ion exchange between calcium ions and heavy metal ions. The surface complexation also played an important role in the Cu(Ⅱ) ion adsorption. The improved Cu(Ⅱ) ion removal efficiency was due to the more functional groups available and the nano-channels formed by CNTs which
increased their surface complexation capability and ion exchange capacity between calcium ions and Cu(Ⅱ) ions. The alginate/CNT/maghemite composite beads not only obviously increased the Cu(Ⅱ) adsorption capability due to the CNTs immobilized in alginate, but also made the adsorbent be removed easily from the effluent solution using magnetic separation methods to avoid the secondary pollution caused by the nano-sized CNTs.
The composites of alginate, CNTs, and iron(Ⅲ) oxide were prepared for the removal of copper ions and the easy recovery of composites after usage. Both alginate and CNTs improved the Cu(Ⅱ) ion removal efficiency by the synergistic effect of high absorption from surface complexation and ion exchange. The iron(Ⅲ) oxide was introduced in the alginate/CNT composites for the easy magnetic recovery after usage based on its magnetic property. Both the improved Cu(Ⅱ) ion removal efficiency over 60% and the easy magnetic recovery of composite were achieved by using alginate/CNT/maghemite composite beads.