With the remarkable properties of carbon nanotubes(CNTs), studies on enhancing the properties of epoxy composites by adding CNTs have been promoted rapidly in recent years. Epoxy resins, especially bisphenol A type epoxy resins, are viscous liquids at room temperature resulting in poor wettability to CNTs in general. Therefore,the addition of solvent and/or surfactants to epoxy resin has been widely used to reduce the viscosity to improve the dispersion of CNT in polymer matrix [1-6]. Epoxy resins are undergone cure reactions after the curing agents are added,there are several ways how to incorporate CNTs into epoxy resins. Usually CNTs have been introduced into epoxy resins via solvent methods, where CNTs are first dissolved in a solvent followed by mixing into epoxy resin. These methods ensure sufficient wetting of CNTs and offer convenient ways of introducing CNTs into epoxy resins, the need to eliminate the solvent before cure reaction may cause environmental problems as well as the process complexity.
Recently, supercritical fluids (SCF) have been emerging as an environmentally friendly solvent and blowing agent in a wide range of applications [7,8]. Supercritical fluids are known to be attractive alternatives to incompressible organic liquid solvents, since they can have liquid-like dissolving power while exhibiting transport properties of a gas. Supercritical carbon dioxide has received a great deal of attention as a polymerization and processing medium primarily driven by the need for replacing conventional solvents with more environmentally benign and non-toxic,nonflammable and inexpensive ones. However, the practical use of supercritical carbon dioxide has been limited because of the need for high CO2 pressure to dissolve even small amounts of polar, amphiphilic, organo metallic, or high molecular weight polymers [9-13].
In this paper, we report a simple, effective and low temperature route to prepare amine-epoxy adducts (AEA)/thin-multi walled carbon nanotubes (TWCNTs) composite particles using dry processes including mechano-chemical bonding (MCB) process and SCF process. The AEA are typical latent curing agents employed in epoxy based anisotropic conductive films (ACFs) which are useful lowtemperature interconnection materials in microelectronic packaging and display industries [14]. The AEA/TWCNTs composite particles have been used as latent curing agents for urethane modified epoxy resin. Cured multiphase epoxy composites were characterized by dynamic mechanical analyzer (DMA) and their morphologies of fractured samples were investigated by scanning electron microscope (SEM).
TWCNTs, which were synthesized by a chemical vapor
deposition method (Korea University), were used in this study. The diameter and length are about 10~30 nm and 10~50 ㎛, respectively. Ammonium bicarbonate (NH4HCO3) was used to modify TWCNTs. AEA (latent curing agent, average particle size: 2~4 ㎛) were obtained from Ajinomoto. Urethane modified epoxy (UME-305) and carbon dioxide (99.999% purity) were supplied by Kukdo Chemical and Shinyang oxygen, respectively.
2.2. Modification of TWCNTs by ball milling process
In order to ensure sufficient dispersion in polymer matrix, TWCNTs were treated by ball milling in the existence of ammonium bicarbonate (NH4HCO3) [15,16]. A cylindrical ball milling vessel was made of aluminum oxide (50 mm in inner diameter, 80 mm in height), which contained 20 ZrO2 milling beads, each of the beads with diameter of 10mm. The vessel containing 1.58 g (0.02 mol) of NH4HCO3 and 0.25 g of TWCNTs were rolled at a speed of 250 rpm for 2 h. After ball milling, the remaining gases in the vessel were removed in a vacuum oven at 100℃ for 24 h. The modified TWCNTs containing amine and amide functional groups were denoted “amide-thin-multi walled carbon nanotubes (a-TWCNTs).
2.3. Preparation of AEA/a-TWCNTs composite particles by supercritical fluid process
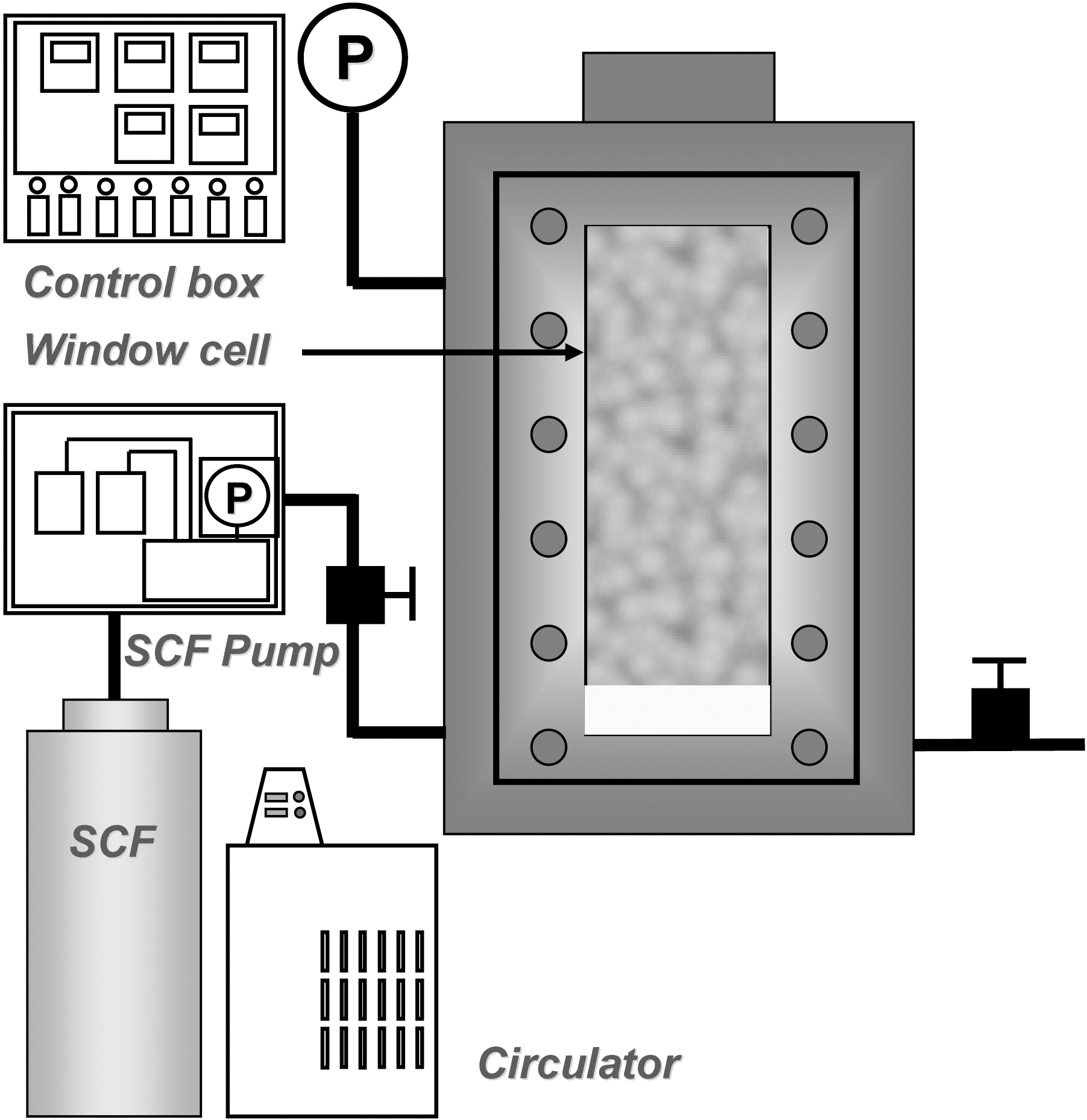
The preparation of composite particles was conducted in a 150-mL stainless steel high-pressure cell equipped with a magnetic stirring bar. Sapphire window on the cell allows
visual observation of the mixture. Fig. 1 shows the apparatus for preparing AEA/a-TWCNTs composite particles by using supercritical fluid process. AEA and a-TWCNTs were separately added to the cell. The cell was then filled with carbon dioxide until the pressure became 70 bar and then heated to 80℃. When the pressure was reached to 250 bar due to heating, the cell was allowed to undergo stirring for 30 min. After the cell was cooled to room temperature, the carbon dioxide was slowly vented to obtain composite particles.
2.4. Preparation of AEA/a-TWCNTs composites in dry mechano-chemical process
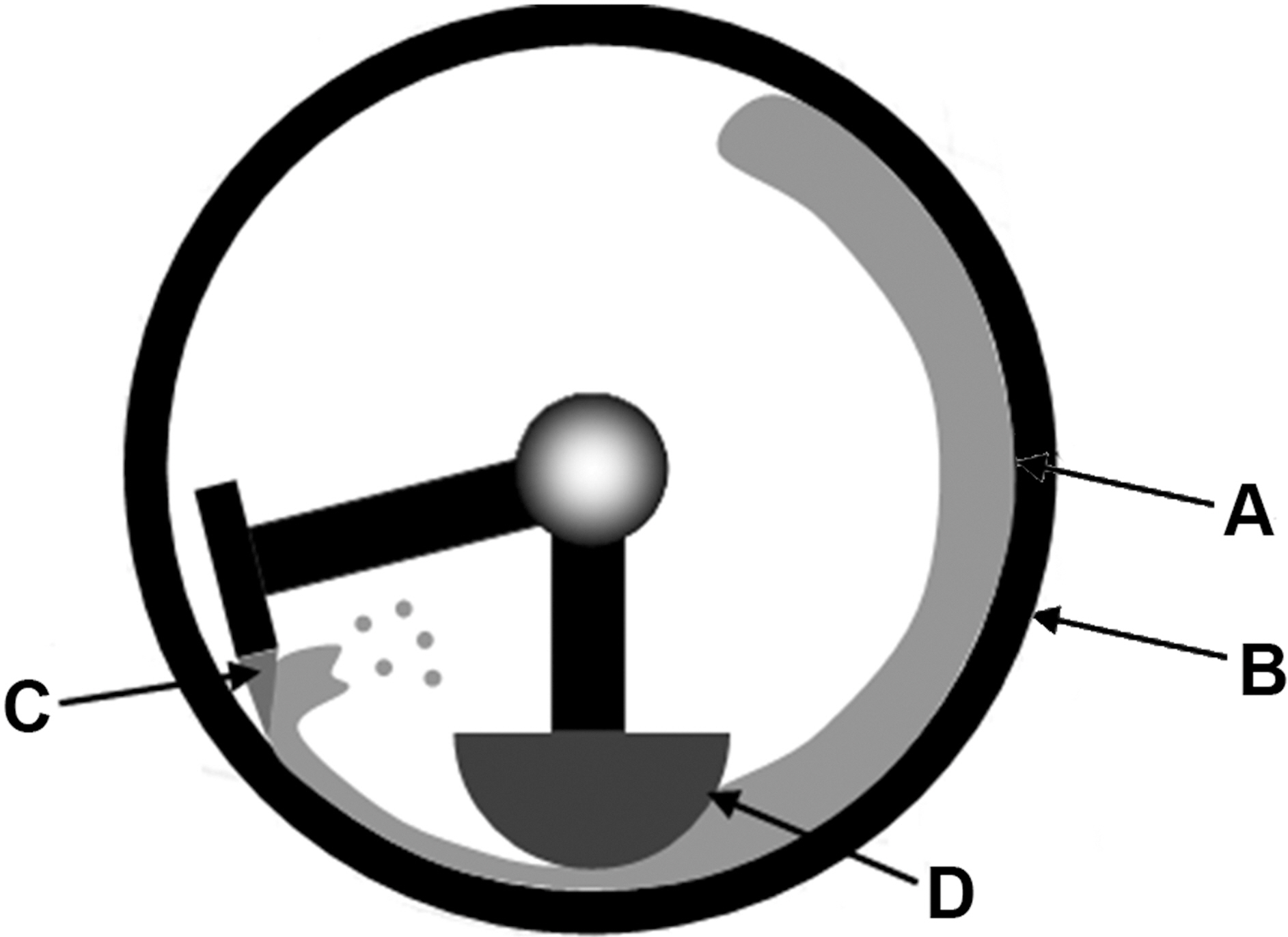
Dry mechano-chemical bonding process was performed with Mechanofusion® AMS (Hosokawa Micron, Japan). Fig. 2 is a simple diagram of an apparatus for MCB process. First, a-TWCNTs and AEA were put into chamber. The ratio of TWCNTs to AEA by weight is 0.0178. The gap between the press head (Fig. 2-D) and the rotor (Fig. 2-A) wall surface was 1 mm. The rotation speed was 2500 rpm and process was conducted for 5 min.
2.5. Preparation of epoxy/AEA/a-TWCNTs composites
The epoxy composition containing 0.50 wt% TWCNT was prepared by high speed paste mixer (Twinky, Japan) without any solvent. The composite was cured in an oven for 3 h at 180℃.
Dispersion state of TWCNTs in cured epoxy resins was visualized by SEM (JEOL-6700F) of fractured surface of the samples. The storage modulus and glass transition temperature of the composites were characterized by DMA (TA instruments,
DMA Q-800) using a dual cantilever bending system operating at a frequency of 3 Hz. Measurements were conducted from room temperature to 180℃ at the scan rate of 2℃/min.
DMA was used to measure the themomechanical properties
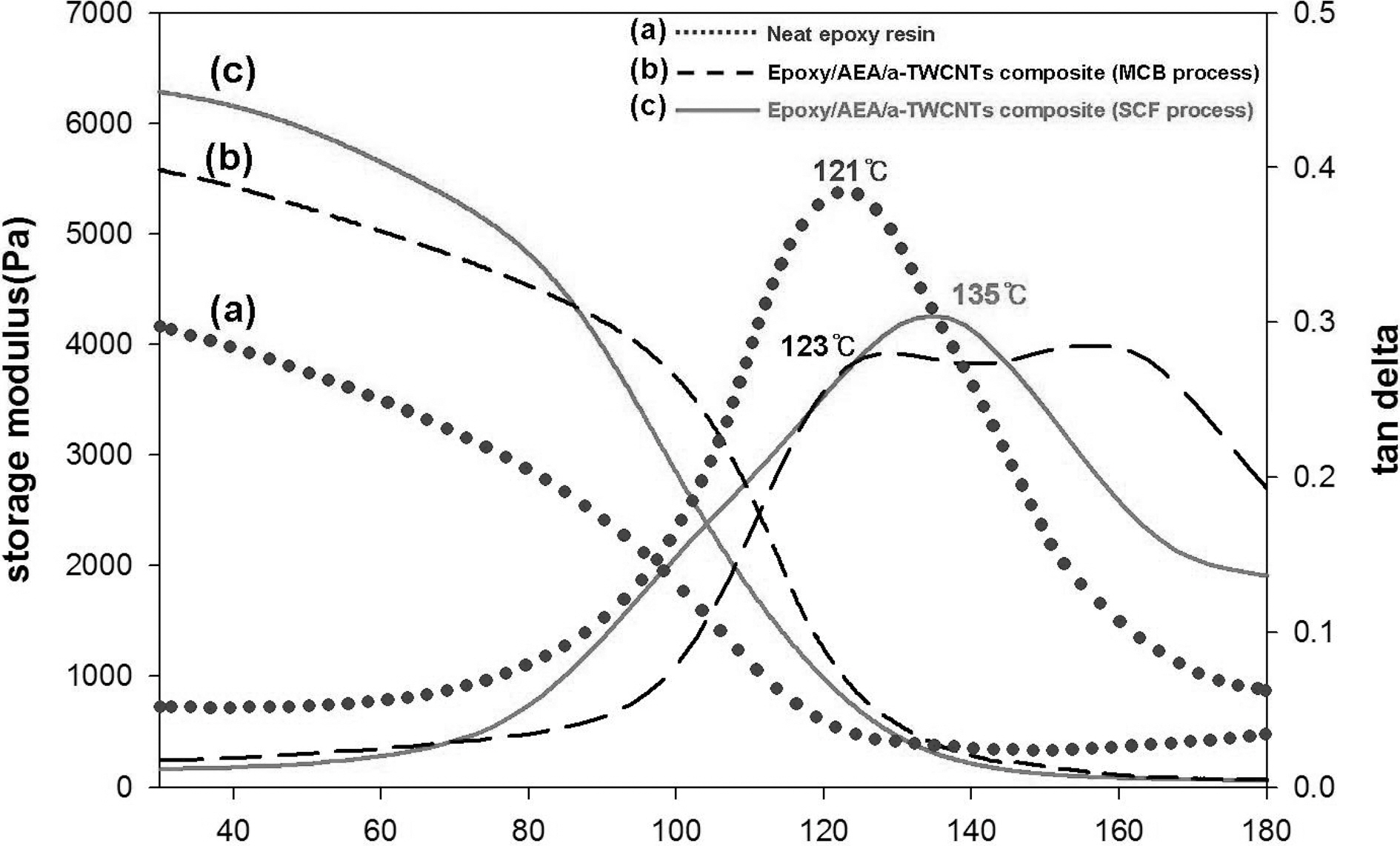
of neat and a-TWCNTs filled epoxy resins. Fig. 3 shows the temperature dependence of storage modulus and tanΔ of neat and epoxy/AEA/a-TWCNTs composites containing 1.75 wt%of a-TWNTs. The storage moduli of the epoxy/AEA/a-TWCNTs composites increased due to the reinforcing effect of the nanofillers, when compared to that of neat epoxy resin. The glass transition temperature, Tg of neat epoxy resin estimated from peak temperature of tanΔ was 121℃. Incorporation of a-TWCNT into epoxy resin via curing agents is found to be highly effective in increasing Tg of resulting epoxy composites. For the cured epoxy/AEA/a-TWCNTs composites wherein composite particles are prepared by SCF process, Tg is as high as 135℃. In case of the cured epoxy/AEA/a-TWCNTs composites where in composite particles are prepared by MCB process, there seem double peaks in tanΔ curve, one is at 121℃ slightly higher than that of neat resin and the other at as high as 157℃. This double peak behavior may arise from the inhomogeneity in crosslinking density caused by the introduction of a-TWCNT affecting the overall cure behavior, though this interpretation needs further investigation. Increased Tg of the epoxy resin filled with a-TWCNTs suggested that the incorporation of nanofillers restrict segmental motions of epoxy resin at the interface or may induce more crosslinking around nanofillers.
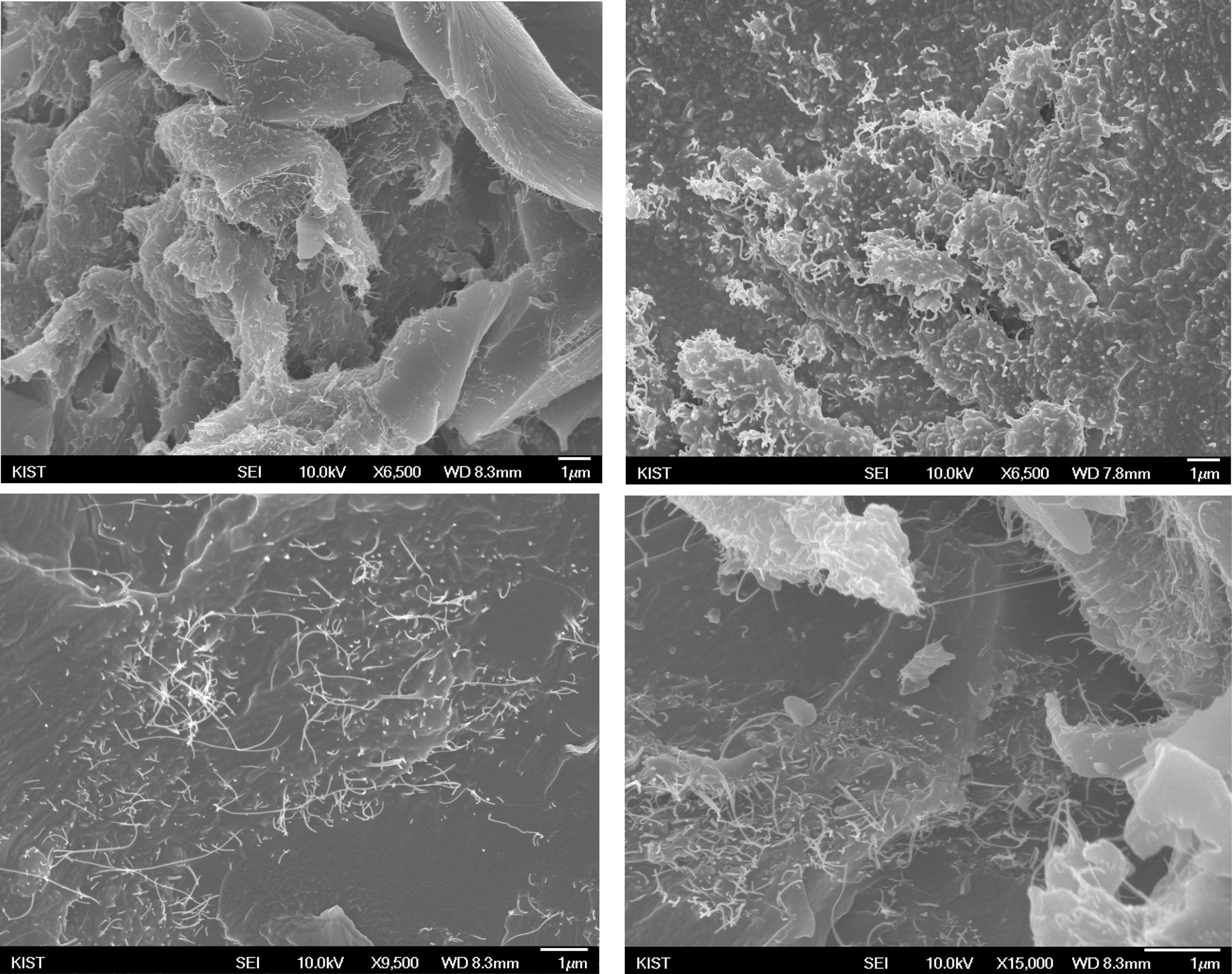
The fracture morphologies of a-TWCNTs/AEA/epoxy composites were shown in Fig. 4 and Fig. 5. Large agglomerates of a-TWCNTs are observed for epoxy/ TWCNTs/AEA composites wherein composite particles are prepared by MCB process (Fig. 4), whereas these agglomerates are almost absent for those wherein SCF prepared TWCNTs/AEA composite particles are used as curing agents. Therefore, SCF process seems to be more efficient than MCB process for preparing CNT/AEA composite particles where nanofillers are well dispersed in epoxy matrix. In addition, the high degree of CNT dispersion observed in the epoxy resin cured by TWCNTs/AEA composite particles prepared by SCF process lead to better thermal and mechanical properties than those cured by TWCNTs/AEA composite particles prepared by MCB process. However, very high Tg value observed in the epoxy resin cured with composite particles prepared by MCB may be attributed to the chemical bonding between a-TWCNT and AEA during MCB process, which further enhance the interactions between naotubes and matrix as a whole, at least locally.
We successfully prepared AEA/TWCNTs composite particles through dry processes including MCB and SCF process. These composite particles were used as curing agents for epoxy resin and found to significantly increase the glass transition temperature of cured epoxy resins in somewhat different manners. When SCF processed composite particles are used as curing agent, significantly increased single Tg (135℃) compared to that of neat resin (121℃) was observed. However, in case of the cured epoxy resin for which MCB processed composite particles are used, double glass transition temperatures (123℃ and 157℃) were observed, for which very high Tg of the two may be attributed to possible chemical bonding between functionalized nanotubes and AEAs. These chemical bonding can be induced by external supply of mechanical forces during composite particle preparation. Morphological observations also generally support above thermomechanical behavior in that nanotubes are well dispersed in SCF case whereas poor dispersion state showing large nanotube agglomerates (or nanotube rich phase) are observed in MCB case.
In conclusion, we introduced new ways of incorporating carbon nanotubes in epoxy matrix by using composite particles containing carbon nanotubes as curing agents. Particularly we found that dry processes including MCB and SCF process provide quite efficient ways of preparing composite particles useful as curing agents for epoxy resin.