The decomposition of several chemical compounds inpine needle litter is a relatively long-term process (Couteauxet al. 1998, McTiernan et al. 2003). The completedecomposition of needle litter takes a number of years.Overall, litter mass loss rates in pine forests vary widely,depending on the climate and litter quality (Couteauxet al. 1995, Berg et al. 1996), and initial concentrationof chemical compounds (Kainulainen and Holopainen2002, Kainulainen et al. 2003, Koide et al. 2005). Coniferousspecies primarily generate sizable quantities ofterpenes (Guenther et al. 1995, Kelkar et al. 2006, Kupcinskieneet al. 2008), which is a complex mixture of terpenesconsisting of monoterpenes, sesquiterpenes, andditerpenes. Although highly volatile, monoterpenes caninitially be present at relatively high concentrations inrecently fallen litter, as has also been shown in previousstudies (Wilt et al. 1993). Additionally, the compositionof monoterpenes changes rapidly during the decompositionof needle litter (Kainulainen and Holopainen2002, Ludley et al. 2008), and some compounds are alsodetected in soils (Wheatley et al. 1996, Asensio et al.2008). The compounds remaining in soil play important
ecological roles in the interactions between living soil microorganisms (Vokou et al. 2002, Hwang et al. 2004, Kim et al. 2006). Many authors (Koide et al. 1998, Kleinheinz et al. 1999, Owen et al. 2007) demonstrated that terpenes from forest -soil extracts and enriched cultures could be used by certain soil microorganisms as a source for growth. Therefore, a considerable change in terpene composition in soil is always accompanied by initial terpene concentrations in needle litter and microbial consumption. However, little is currently known regarding the quantitative and qualitative distribution of terpenes in different soil systems (Wilt et al. 1988, Smolander et al. 2006). Recently, monoterpene concentrations have been reported to be highest in surface litter (Asensio et al. 2008, Ludley et al. 2008). The most abundant monoterpenes were identified as α-pinene, β-pinene, and 3-carene in Pinus sylvestris, Picea abies, and Picea sitchensis (Ludley et al. 2008); α-pinene predominated in the roots and β-caryophyllene predominated in the litter layer from Pinus halepensis trees (Asensio et al. 2008). A comparative study of terpenes concentrations in different soil horizons and changes in terpenes of different species during litter decomposition was previously conducted (Ludley et al. 2009) on the basis of monoterpene contents and distribution in the litters and roots of three conifer species (P. abies, P. sitchensis, and P. sylvestris) via the litter bag method. However, no comparative studies of terpene content have thus far been conducted in the field, because such studies should be conducted with the same coniferous species at the same geographical location on the same series of soils.
This study was conducted in a natural forest in Mt. JeongByeong (35o08′59″ N-35o66′29″ N, 128o45′13″ E- 128o36′19″ E, elevation 567 m above mean sea level) in Gyeongnam Province, South Korea. The characteristics of its climate were mild and continental, and the annual average, maximum month, and minimum month temperatures of this region were 15.09oC, 26.7oC (July) and 6.7oC (January), respectively. Additionally, the annual mean precipitation in 2005 was recorded as 1,827 mm. Each of five 20- to 40-year-old trees of the species P. densiflora, P. thunbergii, and P. rigida, with diameters at breast height of 15-30 cm and heights of 3-10 m, were selected. Fresh (green) and senescent (recently dead and fallen) needles were obtained from each tree. Approximately 30 g quantities of fresh needles were enclosed in litter bags (20 cm × 20 cm) made of polypropylene shade cloth with a mesh size of 0.5 mm (Fig. 1). The litter bags were placed in the same plots in which the leaves were collected, and positioned at each of the surface litter layers and at a depth of 20 cm beneath the litter layer. The bags were attached to the soil with metal pins to prevent movement. The decomposition study encompassed one year, from April 2004 through April 2005. The bags were sampled 10 times at monthly intervals. On each sampling occasion, some of the needles from each of 9 bags were collected and placed in plastic bags, then carried to the laboratory for analysis of mono- and sesquiterpenes.
Foreign materials attached to the outsides of the bags were removed carefully; decaying needles were prepared for analysis by gently removing the soil. Three grams of needles were then collected from each litter bag, placed in a mortar with pure sand and 1% tetradecane as an internal standard, then extracted with 30-50 mL of pen
tane. After extraction, extracts from the fresh and decaying needle samples were concentrated under nitrogen gas to 10 mL and 1.0-5.0 mL, respectively. One μL aliquot of each sample was injected into a gas chromatography-mass spectrophotometer (HP 5890II-5972 MS; Hewlett Packard, Wilmington, DE, USA) with an FID detector. A HP-5 capillary column (i.d. 0.25 mm, 30 m long) was used for the FID. The GC/FID oven program was initially set to 37°C for five minutes, increased to 180°C at a rate of 5°C /min, and then by 20°C /min up to 320°C.
Terpene calibrations were conducted via comparisons with the spectral data of the internal spectral library of the instrument (Wiley Library, ver. 7.0) and retention times, based on references. The concentration of peaks at selected retention times were estimated from peak areas using an internal standard curve for tetradecane. The differences in terpene concentration during the decomposition periods were analyzed for each species via analyses of variance (ANOVA).
>
Terpenes in green and senescent needles from different pine species
The percentages were determined via the area normalization method, and the obtained results were submitted for statistical analysis. We discussed the components with retention times of less than 30 minutes, since the peaks of components with retention times higher than 30-35 minutes were largely unstable. The components with concentrations in excess of 0.05% were expressed as “very low” or “trace”, although many components were detected in all three species.
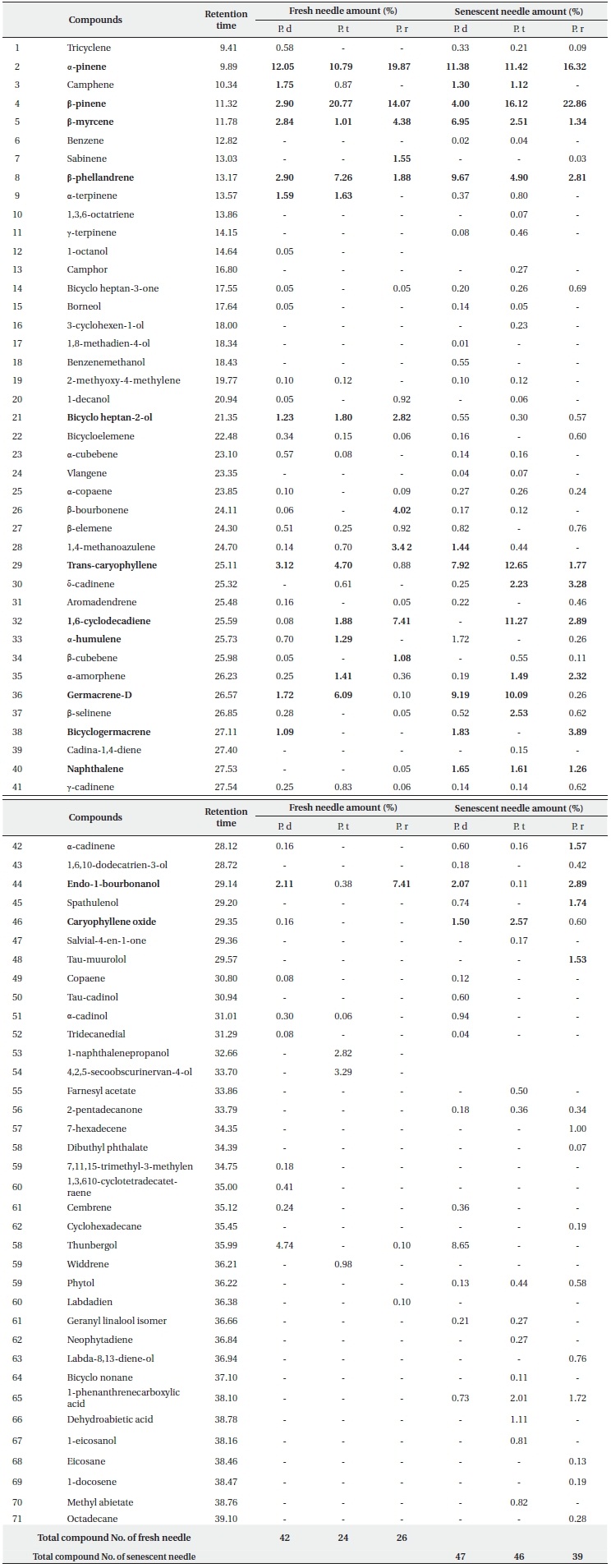
Essential oil compositions in the fresh and senescent needles from 3 pine species are shown in Table 1. Seven monoterpenes and 10 sesquiterpenes were identified as components with concentrations higher or near 1% in fresh needles from three pine species. Among the monoterpenes, α-pinene (10.79 -19.87%), β-pinene (2.90-22.86%), β-myrcene (1.01-6.95%), β-phellandrene (1.88-9.67%) are the major ones, and occur commonly in all three pine species. Among the monoterpenes obtained from P. densiflora fresh leaves and senescent needles, α-pinene (12.05% and11.38%, respectively) is the principal one, followed by β-pinene (2.90% and 4.00%, respectively). However, in the fresh and senescent needles of P. thunbergii, β-pinene (20.77% and 16.12%, respectively) was detected as the primary monoterpene, followed by α-pinene (10.79% and 11.42%, respectively). The component found at the highest levels in the fresh needles from P. rigida was α-pinene (19.87%), followed by β-pinene (14.07%), but in the senescent needles β-pinene was the
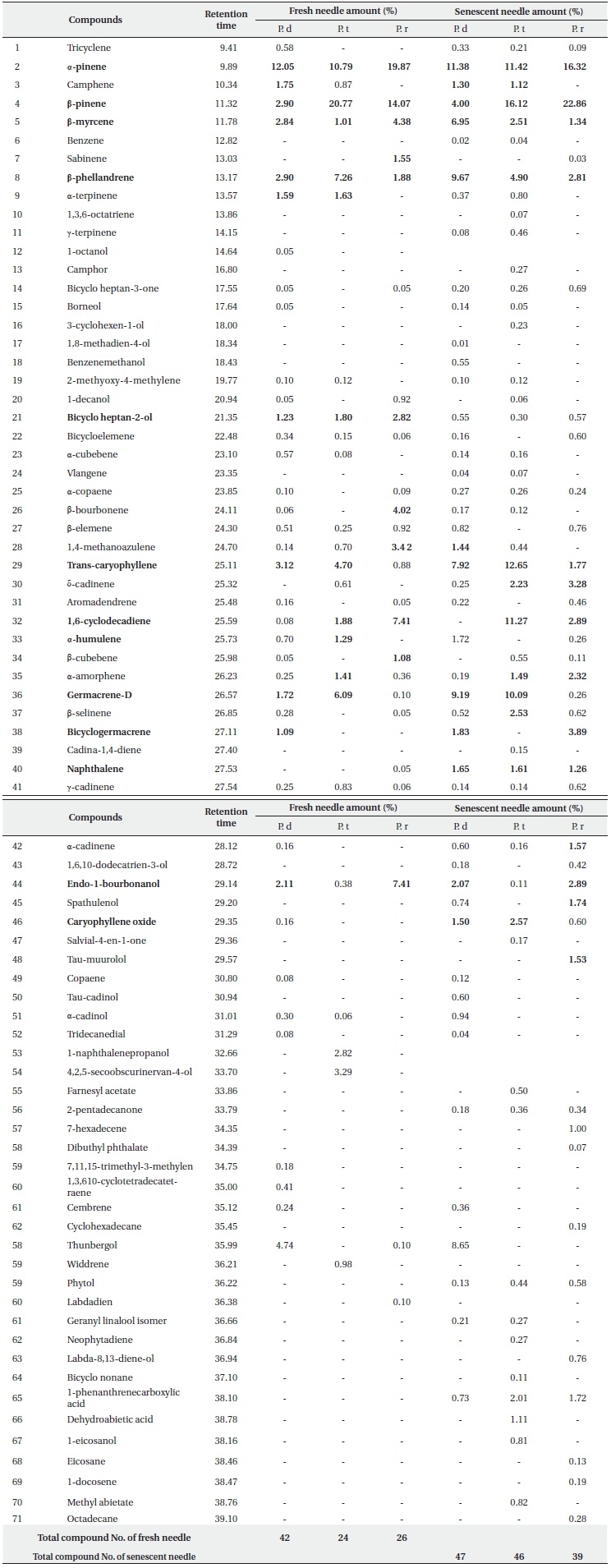
Composition of the total essential oil from fresh and senescent needle extracts of P. densiflora (P. d), P. thunbergii (P. t), P. rigida (P. r). Only compounds found at level > 0.05% are included (N = 3)
most abundant component (22.86%). The results reported by several researchers (Yu et al. 2004, Lee et al. 2005, Bao et al. 2008), were consistent with our results for P. densiflora. They reported that the content of α-pinene was relatively higher than β-pinene in P. densiflora leaves. On the other hand, different results were reported by Kang and Kim (1997), Tani et al. (2002) and Kim and Shin (2005) for P. densiflora leaves. Kang and Kim (1997) reported that the concentrations of β-pinene were higher than the concentrations of α-pinene in all three species, and Tani et al. (2002) suggested that the most abundant monoterpenes from P. densiflora in Japan were β-phellandrene (37.4%), although the content of α-pinene (28.7%) was higher than that of β-pinene (19.8%). The primary monoterpene components of P. densiflora fresh needles detected by diethyl ether at pH 3.6 were α-ocimene (29.3%), sabinene (10.9%), β-myrcene (9.6%) and β-pinene (4.3%) (Kim and Shin 2005). Ludley et al. (2009) also insisted that there were typically two dominant monoterpenes (α- and β-pinene) in the green needles from conifer species (P. abies, P. sitchensis, and P. sylvestris). As many researchers (Janson 1993, Lee et al. 2005, Randrianalijaona et al. 2005) have reported, our results also suggested that the observed differences between the primary components and the concentrations of essential oil from the needles of Pinus species were based on variations in geographical conditions, sampling time, and extraction methods. As can be seen in Table 1, fresh needle monoterpenes from P. densiflora and P. thunbergii as compared to P. rigida were characterized by high contents in terpenic compounds, including camphene (1.75%, 0.87% vs. 0%, respectively) and α-terpinene (1.59%, 1.63% vs. 0%, respectively). On the other hand, tricyclene (0.58%), 1-octanol, borneol and 2-methoxy-4-methylene were detected only in P. densiflora and sabinene (1.55%) was detected only in P. rigida. Among the principal terpenes in fresh needles, β-myrcene, β-phellandrene, and α-terpinene, bicycle-heptan-2-ol, α-copaene, α-cadinene, and trans-caryophyllene did not differ significantly (P > 0.05%) between the three pine species. Camphene, bicycle-heptan-2-ol, and naphthalene in senescent needles did not differ significantly (P > 0.05%) among all three pine species. Additionally, the contents of most terpenes differed significantly (at 0.05%) among the three pine species. The difference in terpene composition between species might be based on systematic and genetic differences (Scrivanti et al. 2009), owing to the fact that P. thunbergii and P. densiflora are native species, but P. rigida is an induced species. Therefore, a systematic study will be necessary to further investigate terpene composition distances and/or correlations among species.
Bicyclo heptan-2-ol (0.30-2.82%), trans-caryophyllene (0.88-12.65%), germacrene-D (0.10-9.19%), 1,6-cyclodecadiene (0.08-11.27%) and endo-1-bourbonanol (0.11-7.41%) were the principal sesquiterpenes detected commonly in the fresh and the senescent needles of all 3 species. Among the fresh needle sesquiterpenes detected in P. densiflora, trans-caryophyllene (3.12%) was the most abundant compound, along with germacrene-D (6.09%) in P. thunbergii and 1,6-cyclodecadiene (7.41%) and endo-1-bourbonanol (7.41%) in P. rigida. Germacrene-D (9.19%) was the highest content compound in the senescent needles from P. densiflora, trans-caryophyllene (12.65%) in the senescent needles from P. thunbergii, and bicyclogermacrene (3.89%) was the highest content compound in P. rigida.
There have been many studies regarding monoterpene contents in coniferous trees (Wilt et al. 1993, Asensio et al. 2008), but few studies of sesquiterpene contents and profiles for coniferous plants have been conducted thus far. A previous study performed by Kim and Shin (2005) showed that β-caryophyllene (8.0%), β-cadinene (7.3%), α-terpinolene (4.9%) and 2-hexanal (4.3%) were the principal sesquiterpene compounds of P. densiflora needles in Korea, but this was not consistent with our results. However, no studies have been done thus far comparing sesquiterpene contents and profiles from needles of P. thunbergii and P. rigida in Korea. Three other studies on the sesquiterpene contents of Pinus species are worth considering in this regard (Barnola and Cedeno 2000, Dob et al. 2005, Asensio et al. 2008). The primary sesquiterpene compounds of needles from Pinus caribaea and P. halepensis were identified as β-caryophellene and α-humulene, although no comparative studies were conducted. Kupcinskiene et al. (2008) reported that (E)-caryophellene and δ-cadinene were the highest-level sesquiterpenes from P. sylvestris.
As is shown in Table 1, the total components detected from senescent leaves of three pine species were increased as compared to the components acquired from fresh needles. A total of 47 components were detected for P. densiflora, 46 for P. thunbergii, and 39 for P. rigida, which were obtained via the addition of new compounds to the data of the fresh needle components. These results showed that the biological degradation of monoterpenes and sesquiterpenes was induced by microorganisms such as fungi and bacteria (Harder and Probian 1995, Cleveland and Yavitt 1998, Osono et al. 2006). Harder and Probian (1995) previously performed an investigation into the anaerobic degradation of natural monoterpenes with Pseudomonas citronellois. They determined that the consumption of monoterpenes, including linalool, menthol, menth-1-ene, α-phellandrene, limonene, 2-carene, α-pinene, and fenchone, depended on the presence of living microorganisms and nitrates. They also reported that geraniol and geranial were generated from linalool via biotransformation. However, it is difficult to evaluate its correlation with individual compounds in terms of microbial activity.
The content ratios of δ-cadinene, bicyclogermacrene, naphthalene, and caryophyllene oxide in senescent needle extracts were higher than those of fresh needle extracts in all three species. In particular, trans-caryophyllene and germacrene-D compositions of senescent needles of all three types of pines were elevated as compared to those detected in the fresh needles. The proportional content of 1,6-cyclodecadiene detected in senescent needles from P. thunbergii was approximately six times higher and the germacrene-D content from P. densiflora was approximately five times higher than that of the fresh needles. Bicyclogermacrene (3.89%) and δ-cadinene (3.28%) from P. rigida were detected at markedly higher levels in the senescent needles. The increased levels of sesquiterpene compounds in the senescent needles indicate that those compounds become more abundant during the decomposition process. Additionally, although the relevant data are not shown in this study, the ratio of total sesquiterpenes was increased above that of total monoterpenes during decomposition in all three species. Li et al. (2008) asserted that sesquiterpenes might originate from decaying needle litter or fungal hyphae. Our results also revealed that elevated sesquiterpene levels and/or new sesquiterpenes might be produced and consumed by microbial activity in soils (Schade and Custer 2004, Sidorenko and Buzoleva 2008), and may have occurred as the result of litter-fall inputs from the aboveground needle parts, as was suggested previously by Asensio et al. (2008).
Terpenes loss from needles during decomposition
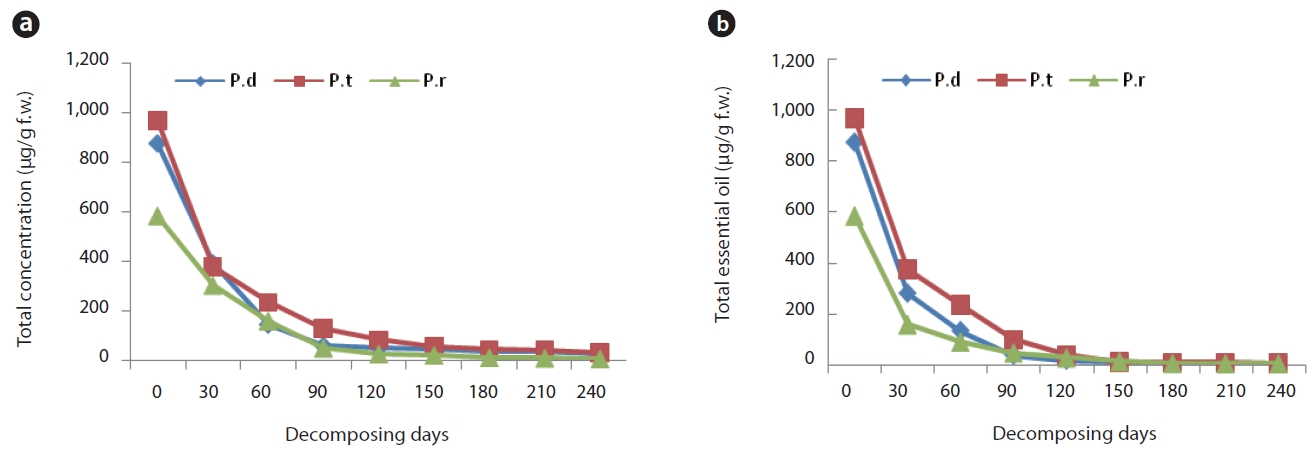
Kim et al. (2006) previously conducted a study of monoterpene concentrations in litterbags over a one-year period. According to that report, total monoterpene concentrations in all three species evidenced a rapid decrease for the first 90 days, and some monoterpenes remained at a low concentration throughout the entirety of the experimental period. The quantities of main essential oil loss from the remaining litter at 30-day intervals are provided in Fig. 2a and 2b. The concentration of essential oils in the litters of all three species as well as the monoterpene concentrations evidenced a rapid reduction for the initial 90 days after litter decomposition. We noted some slight differences in the soil horizons and the species. The total essential oil concentration in the litter of P. densiflora and P. thunbergii, respectively, decreased to 44.7% (390.7 μg/g f.w.) and 43.1% (377.4 μg/g f.w.) of the initial litter concentration determined in the first sampling. The total essential oil concentration in litter of P. densiflora decreased to 4.82% (24.6 μg/g f.w.) by day 120 and that of P. thunbergii was reduced to 4.92% (29.0 μg/g f.w.) by day 150. Concentration of the total essential oil in the needle litter of P. rigida evidenced a sharper reduction. At the first sampling (after day 30), the essential oil concentration of needle litter declined to 35.5% (301.8μg/g f.w.) of the initial content, and to 5.59% (5.3 μg/g f.w.) by day 60. However, the initial concentrations in the freshly fallen litter of P. rigida were far lower than those of the other two species (583 μg/g f.w. compared to 875 μg/g f.w. and 968 μg/g f.w. in P. densiflora and P. thunbergii, respectively) (Fig. 2a). These results are consistent with a study conducted by Ludley et al. (2009), in which it was reported that the total monoterpene concentrations from P. abies, P. sitchensis, and P. sylvestris declined rapidly to approximately 4% of the initial concentration in 6 months.
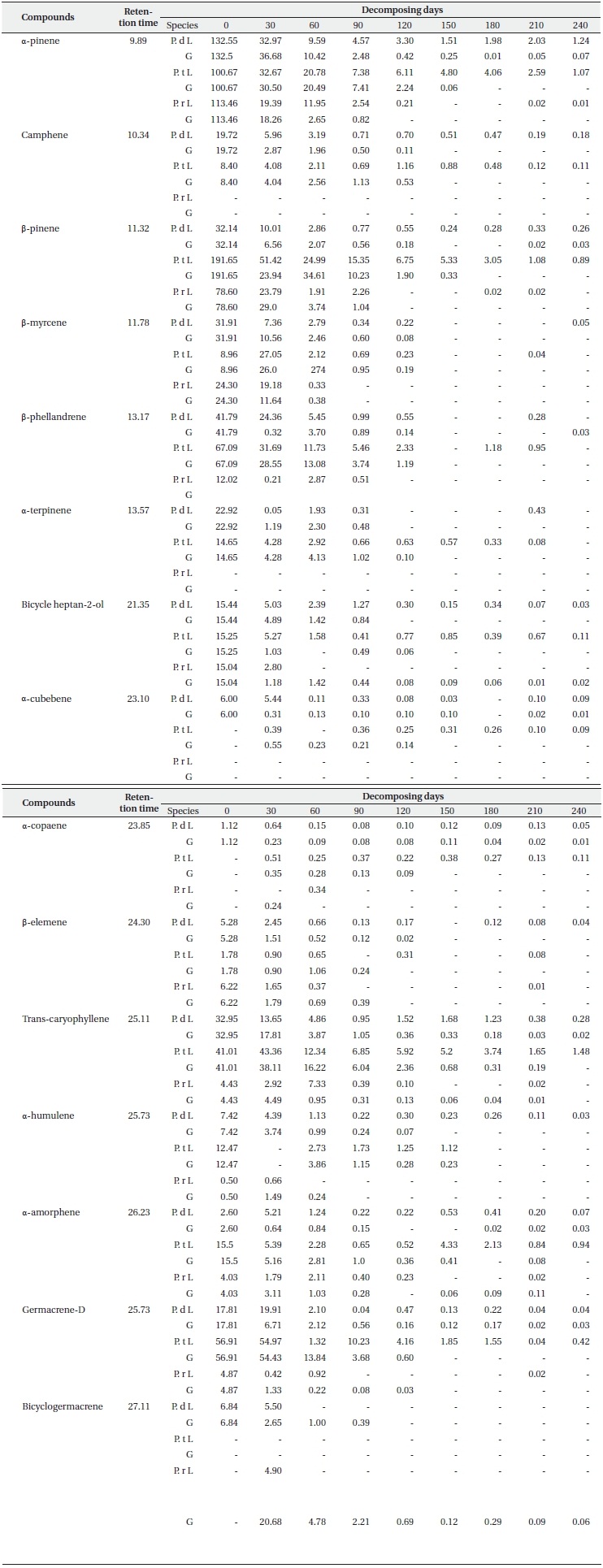
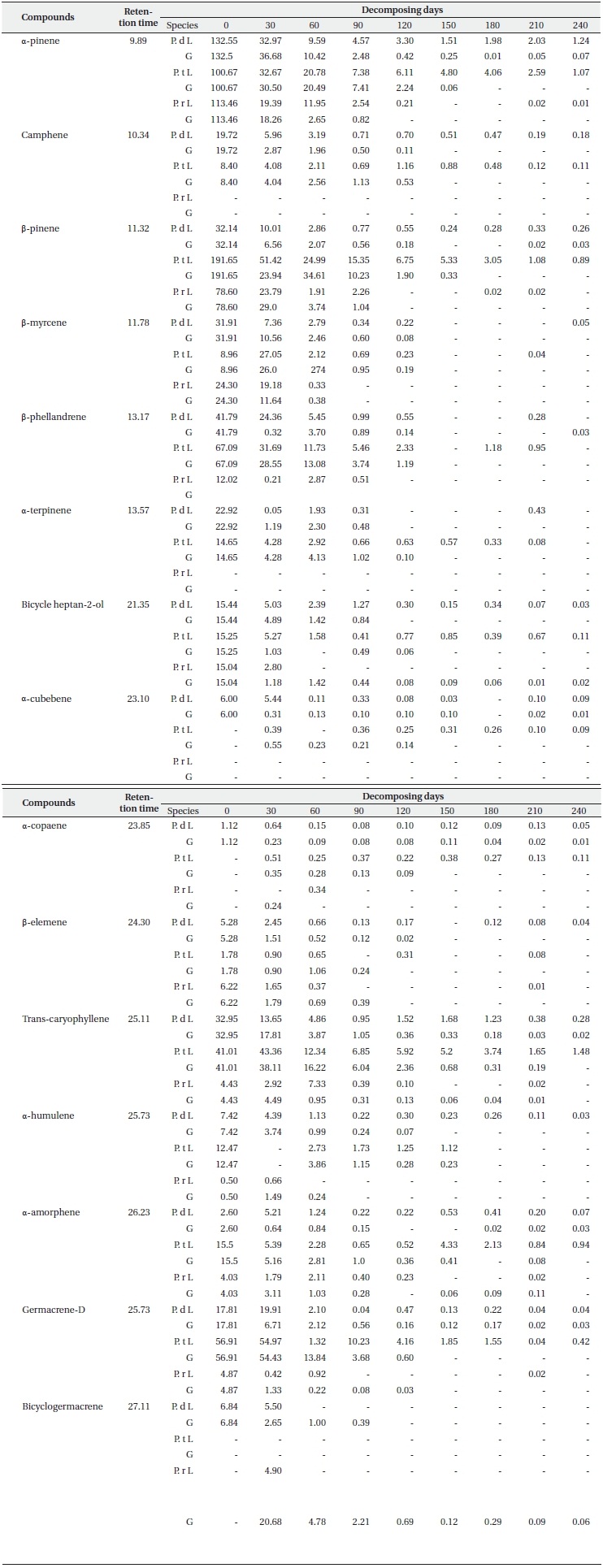
Concentration of main terpenes (μg/g f.w.) in litter (L) and ground layers (G) of Pinus densiflora (P. d), Pinus thunbergii (P. t), and Pinus rigida (P. r) during decomposition (N = 3)
In the litter under layers, the total essential oil concentration in the P. densiflora, P. thunbergii, and P. rigida samples, respectively, decreased to 32.1% (280.6 μg/g f.w.), 43.0% (376.4 μg/g f.w.) and 17.9% (156.4 μg/g f.w.) of the initial litter concentrations determined in the first sampling. The total essential oil concentration in the remaining litter of P. densiflora and P. rigida was also greatly reduced; the rate of loss of essential oils was higher than that of the litter layer (Fig. 2b). On the other hand, concentrations in the needle litter of P. thunbergii evidenced a similar rate of loss to the litter layers. Concentrations of P. densiflora, P. thunbergii, and P. rigida in litter layers declined to 2.8%, 3.3% and 0.6% of original litter, respectively, at the end of the sampling period. However, those of all three species in the under layers of the litter were 0%.
All these results demonstrated clear changes in the essential oil contents in needle litter from Pinus species during the decomposition process. Terpene concentration in the litter is susceptible to seasonal changes due to variations in environmental conditions and decay status throughout the year (Kainulainen and Holopainen 2002). Therefore, terpenes concentrations in deeper soil horizons might depend on seasonal patterns of litter-fall inputs from the aboveground parts of the tree to the soil surface. In this study, our results show that the highest rate of loss in concentration from all three species occurred by day 90 (3 months later), which was in about July, since the litter bag experiment began in April. July is a very hot and humid summer season in Korea. It was difficult, in this study, to delineate factors that might have influenced the changes in terpene concentration from Pinus species, but seasonal factors may be one of the more important factors, as previously asserted by Asensio et al. (2008). The rate of monoterpenes loss is thought to be largely related to the initial monoterpene concentration (Ludley et al. 2009). Despite the fact that the initial concentrations in P. densiflora and P. thunbergii litter were higher than that measured in P. rigida litter, the loss rate of essential oil from P. rigida was higher than any of the other samples evaluated. As mentioned above, this might translate to a genetic difference; P. rigida is an induced species, but the others are native.
Each terpene had a small proportion of its original content remaining in the surface litter layer and the litter under layer at the end of the sampling period (Table 2). It was determined that the three types of reducing patterns of each compound depend on the rate of loss of concentration during the decomposition process. One of them is a sharp reduction in the initial period (like α-pinene, β-pinene, β-myrcene, β-phellene, bicycle heptan-2-ol, trans-caryphellene and germacrene-D), another one involves a steady or slow decline (camphene, α-cubebene, α-copaene, β-elememe, α-humulene and α-amorphene), and the final one is a newly detected pattern at low concentrations during decomposition (α-cubebene for P. thunbergii, α-copaene for P. thunbergii and P. rigida, and δ-cadinene and bicyclogermacrene for P. rigida). In particular, the remaining terpene contents in the litter layer from P. rigida disappeared completely at the final sampling days as compared to P. densiflora and P. thunbergii (with the exception of α-pinene). The α-pinene concentrations in the litter layers from P. densiflora and P. thunbergii were 1.24 and 1.07 μg/g f.w., respectively, at the final sampling. This may be attributable to the sizeable quantities of remaining monoterpenes; the trans-caryophellene contents from P. thunbergii (1.48 μg/g f.w) identified it as the most abundant sesquiterpene remaining in the litter until the end of the experiment. Other studies have considered the loss of terpenes from litter of conifer species over time in the field (Kainulainen and Holopainen 2002, Ludley et al. 2009), although no similar studies have been published for P. densiflora, P. thunbergii, and P. rigida. Moreover, little information is currently available regarding the concentrations of each terpene in different soil horizons and decomposition periods. Both of the aforementioned studies reported a rapid initial loss of monoterpenes, which is consistent with the results of the present study. The loss rate of each compound might be related to its initial concentration, as mentioned above. However, we detected significant differences in the loss rates of concentration between the litter layer and the litter under layer (Table 2), although the initial concentrations were the same. Therefore, other factors influencing the rates of terpene loss might have been present during decomposition in this study. These factors include temperature, the difference in the volatility of the dominant compound in the litter, and the availability of compounds as a carbon source for micro
organisms in the soil (e.g. α-pinene, the most abundant compound in the litter of P. densiflora and P. rigida is more volatile than β-pinene, which is the most abundant compound in the litter of P. thunbergii). The loss data of each compound (Table 2) demonstrated that the P. densiflora and P. thunbergii needles in litter decomposed more slowly than those of P. rigida, which may have contributed to the slightly higher amounts of monoterpene than sesquiterpene (except for endo-1-bourbonanol) remaining in the P. rigida litter at the final sampling. The variations in the concentrations of each terpene obtained in the litter bag experiment showed that the majority of terpene contents were lost from the litter of all three species in the litter under layers. This is consistent with the research conducted by Asensio et al. (2008); it suggested that higher terpene concentrations for all compounds from P. halepensis trees were observed in the top mineral horizon, as compared to the low mineral horizon.
In conclusion, the concentration of each essential oil in all three species was clearly shown to be affected by the depth of the layer and the decomposition stage of the needles. There is, therefore, transparently a need for further investigations into the sources of terpenes, particularly on the needle-degrading microorganisms and their physical and chemical factors in soil systems.