There has been growing interest in the application of tissue and protoplast culture techniques in seaweeds for genetic manipulation and micro propagation both in order to improve the aqua cultural crops and to increase the knowledge about the process of differentiation, morphogenesis and regeneration (Yokoyo and Handro 1996). The marine algal biotechnology and the basic knowledge and technique for tissue and cell culture in this field are still in the state of development. For the last several years attempts have been made with seaweed tissue culture, following the success achieved in higher plants.
In seaweeds the first demonstration of totipotency of a piece of tissue was obtained from the work of Chen and Taylor (1978) on commercially important red alga
Among red macroalgae, however, less is known about the effect of carbon sources and plant growth regulators on cell cultures. The ability to assimilate carbon sources has been well documented (Robaina
Plant growth regulators have been studied in relation to their occurrence with in different groups (Hamana and Matasuzaki 1982; Yokoyo and Handro 1996) and their involvement in cell division (Cohen
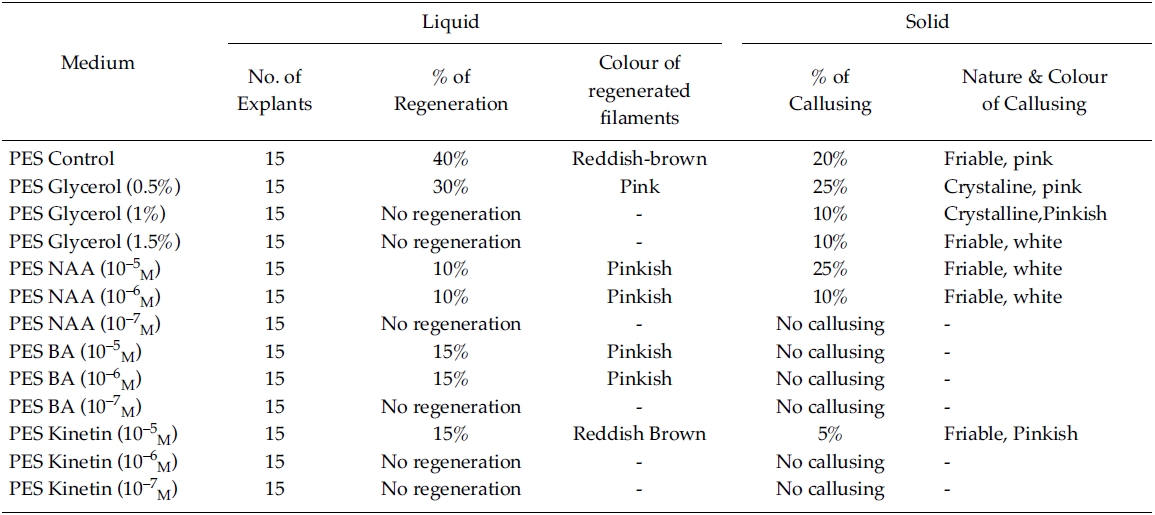
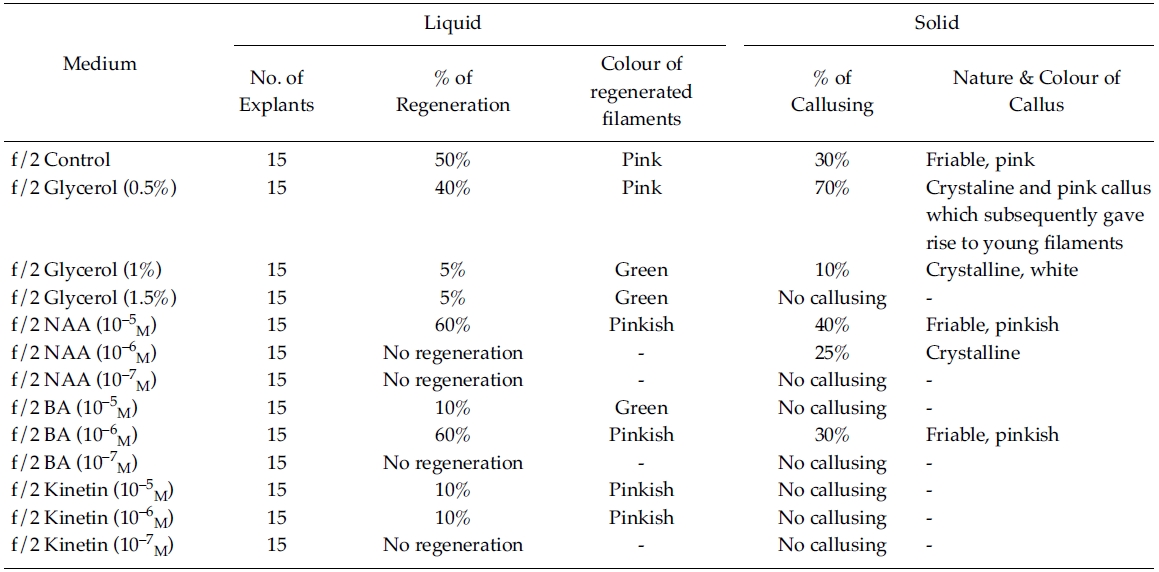
[Table 1.] Effect of phytoregulators on explants (f/2 liquid/solid medium)
Effect of phytoregulators on explants (f/2 liquid/solid medium)
The plants of
The sterilized healthy thalli were kept in 500 ml of autoclaved seawater in a flat-bottom, Florence flask and aerated with compressed air at 20°C temperature and 12:12 light:dark photoperiod as stock culture. The salinity was maintained at 30 ppt throughout the experiment.
Before inoculation thalli were thoroughly cleaned 3-4 times in autoclaved seawater and excised into 3-5 mm in length. Each explant (taken from the central part of thallus )was then wiped gently with sterile filter paper (Whatman no. 1, Maidstone, U.K.) to remove moisture and mucilage exuded from the cut ends.
Three different marine media such as f/2 (Guillard and Rhyther 1962); PES (Provasoli 1968) and ESW (Freshwater and Kapraun 1986) were used, both in solid and liquid form and the pH was adjusted to 8.2. Besides, these media were further supplemented with a number of phytoregulators such as Glycerol (0.5%; 1% &1.5%); Napthalene Acetic Acid (NAA), Benzylaminopurine (BA) and Kinetin all at the concentration of 10?5 M, 10?6 M, 10?7 M. The phytoregulators were added in both solid and liquid media. A control was set for each experiment using autoclaved seawater. Each treatment consisted of three replicates with five explants in each. The explants were cultured in petridish with 20 ml of solid or liquid medium. The medium was solidified using 1.5% agar. Cultures were maintained at 20°C with cool white fluorescent light in 12:12 light:dark photoperiod (Philips fluorescent tubes 36 W/54, 6500 K: 15 μmol m?2 s?1). The results were recorded after every 3 days.
[Table 2.] Effect of phytoregulators on explants (PES liquid/Solid medium)
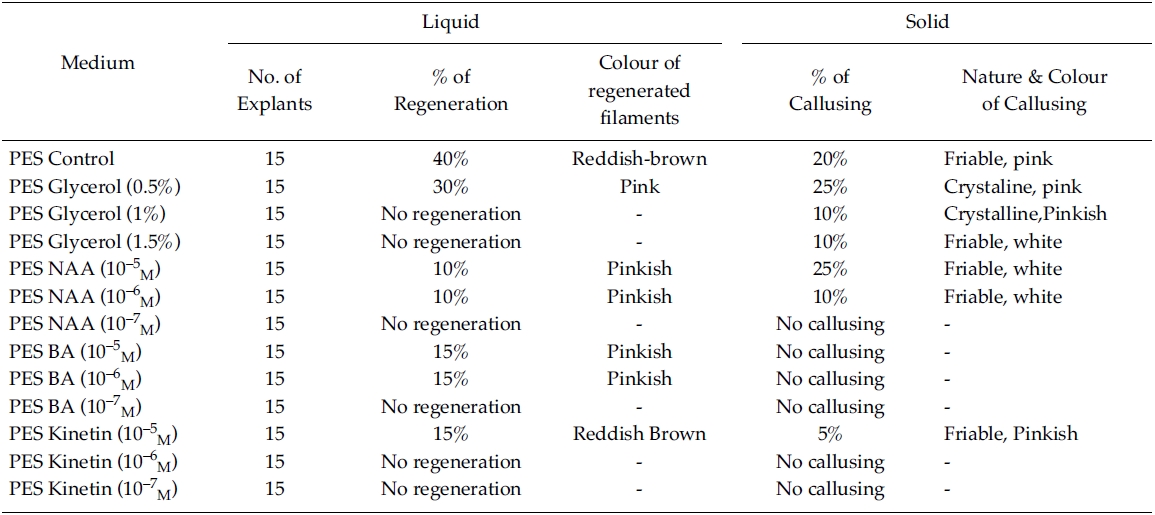
Effect of phytoregulators on explants (PES liquid/Solid medium)
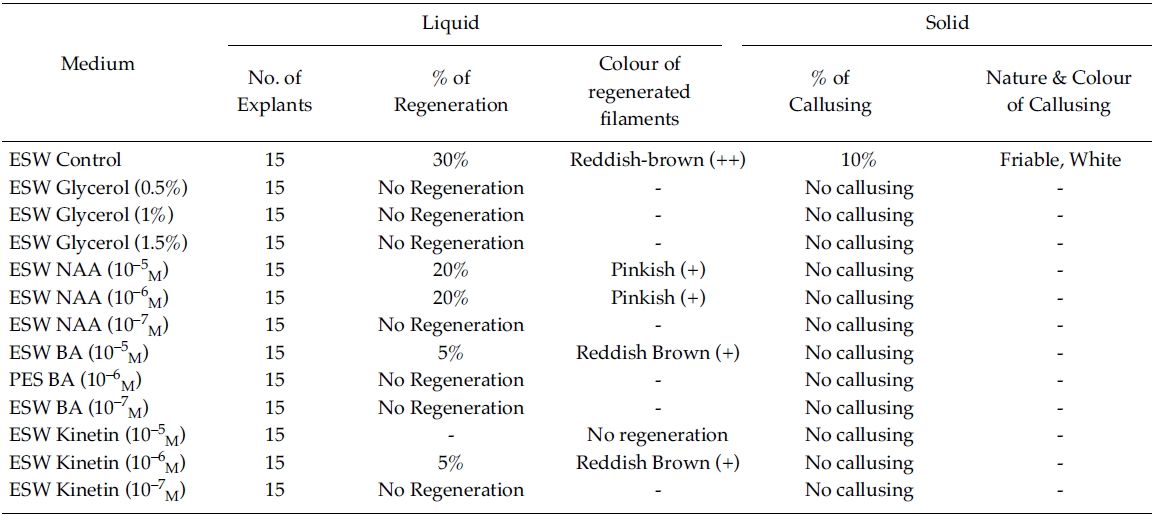
[Table 3.] Effect of phytoregulators on explants (ESW liquid/Solid medium)
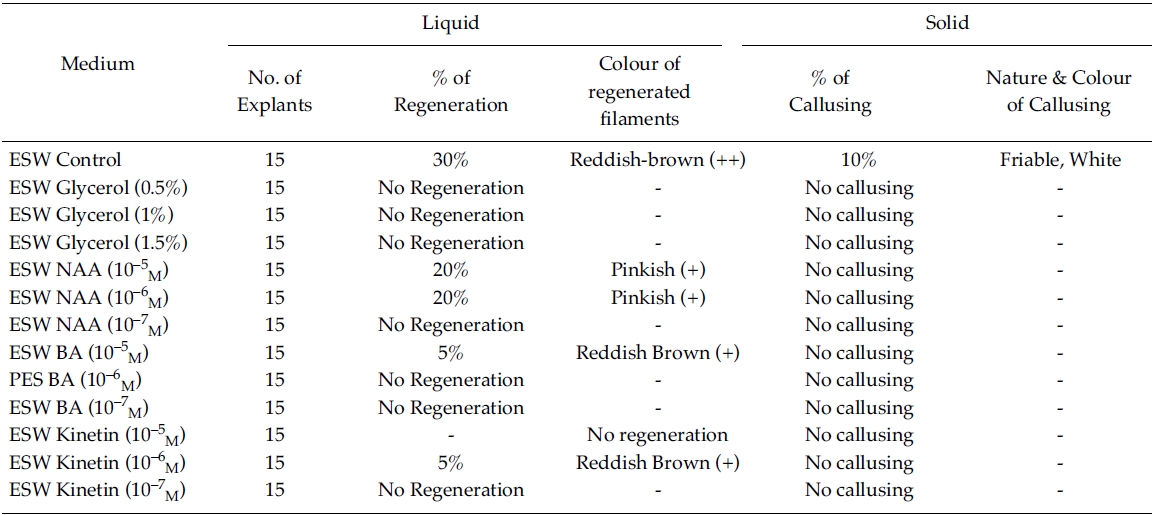
Effect of phytoregulators on explants (ESW liquid/Solid medium)
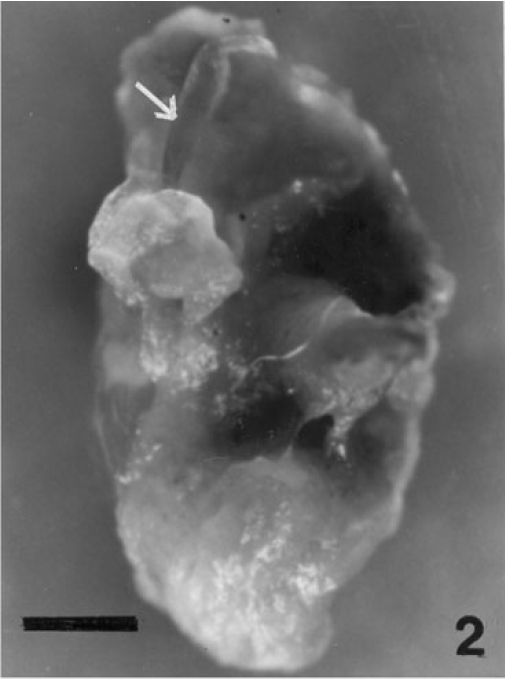
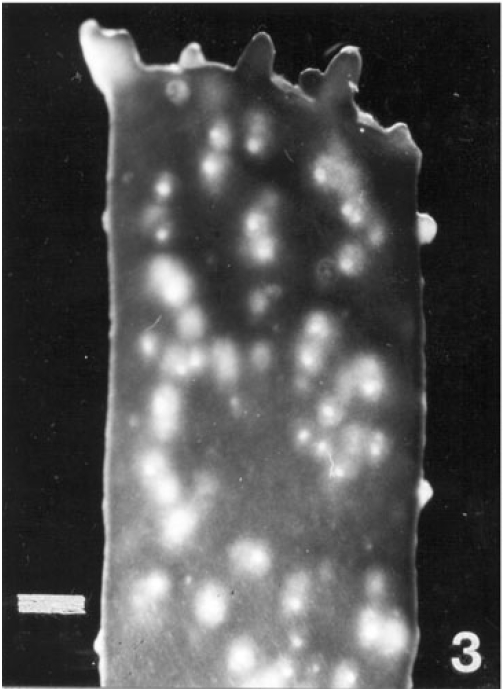
Regeneration of small protrubences was observed from the cut ends of the explants which were inoculated in f/2, PES & ESW liquid media after 3 days of inoculation. Better regeneration of thallus was obtained in f/2 liquid medium (Fig. 3) and PES liquid medium (Fig. 4) after 3 and 7 days of inoculation respectively. No callusing was observed in the explants cultured in the liquid media (Tables 1, 2 and 3).
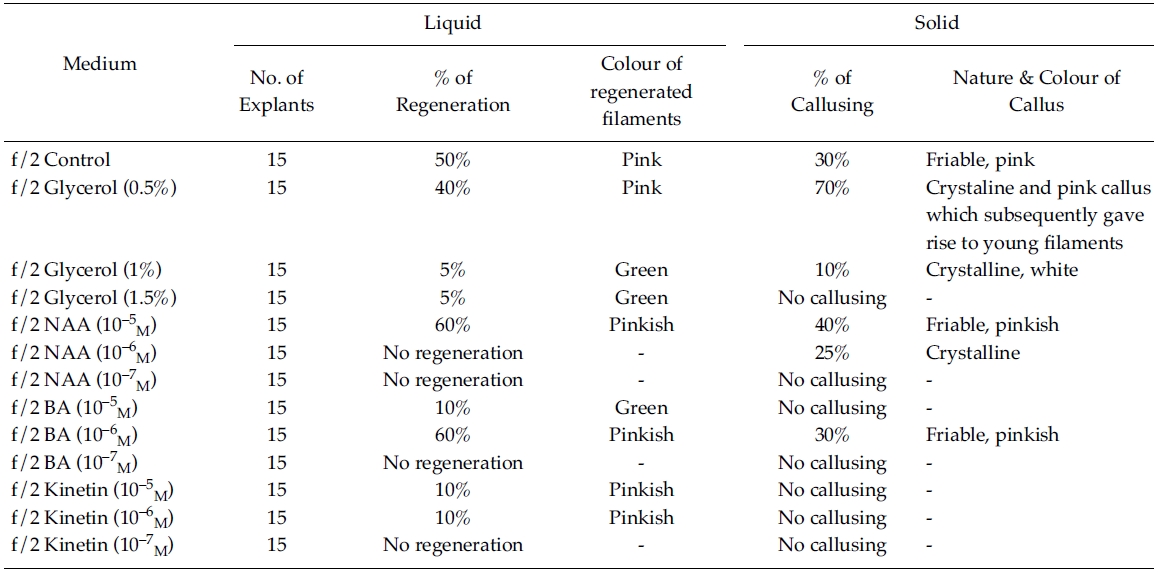
However, callusing was observed after 15 days of inoculation mostly in f/2 & PES solidified media supplemented with various concentrations of phytoregulators (Tables 1 and 2). In the present study no callusing was observed in ESW solid medium supplemented with phytoregulators (Table 3). When the f/2 medium was supplemented with low concentration of glycerol (0.5%) 70% callusing was observed but when the concentration of glycerol was increased the percentage of callusing declined and no callusing was observed when the medium was supplemented with 1.5% glycerol. Similarly lower concentration of NAA 10?5 M and BA 10?5 M produces better callusing compared to the higher concentration of the phytoregulators. The calli were mostly crystalline, friable and pinkish in colour depending on the media (Tables 1 and 2). When the calli were subcultured regeneration of new filaments were observed only in the callus which was cultured in f/2 medium supplemented with 0.5% glycerol after 25 days of subculturing of the callus (Fig. 2) when this regenerated filaments were excised and transferred to f/2 liquid medium new branching was observed after 20 days of inoculation. Extensive branching was subsequently observed and the plants resembled in morphology to the wild plants after 30 days in culture. Thus the whole regeneration with in 90 days from the date of inoculation of explants was completed.
Regeneration is a wide spread phenomenon in seaweeds which demonstrates totipotency of algal cells, provides a mean for studying morphogenetic stages of adventive embryony, and produces correlation growth effects which may be useful for hypothesizing mechanism of regulation of morphogenesis (Buggeln 1981; Baweja
The main objective of the present study was to develop and demonstrate tissue culture methodology in tropical marine algae. In the present study direct regeneration of new plantlets were observed in