Fatigue is a common complaint and affects the quality of life in modern people. Physical stress may induce activation of certain immune cells. Fermented porcine placenta (FPP) has been used to alleviate fatigue. Inflammatory cytokines are produced by physical stress and results in symptoms of fatigue. However, the role of FPP on fatigue-associated inflammatory cytokine production has not been elucidated yet. Thus, we estimated the anti-fatigue effect of FPP and its active components, leucine (Leu) and lysine (Lys) in activated RAW264.7 macrophages and forced swimming test (FST) fatigue animal model. Pretreatment with FPP, Leu, or Lys significantly inhibited the lipopolysaccharide (LPS)-induced tumor necrosis factor-α, interleukin (IL)-1β, and IL-6 production without inducing cytotoxicity on LPS-stimulated RAW264.7 macrophages. FPP, Leu, or Lys inhibited the production of nitric oxide and downregulated the expression of inducible nitric oxide synthase on LPS-stimulated RAW264.7 macrophages. Furthermore, caspase-1 activities increased by LPS were significantly reduced by FPP, Leu, or Lys. In the FST, inflammatory cytokine levels of the mice administrated with FPP, Lys, and Leu were significantly reduced compared with the control group at 21 days. Collectively, these results show that anti-fatigue effect of FPP and its active components, Leu and Lys might be derived from the down-regulating of inflammatory mediators.
Fatigue is a state of mental and physical weakness and declined strength accompanied by a feeling of sleepiness, irritability, and weariness, with a cognitive component (Stebbings and Treharne, 2010). Epidemiological surveys have shown that up to 50% of the general population suffers from fatigue-like symptoms throughout their lifetime. Subsequently, a fifth of these sought medical treatment (Afari and Buchwald, 2003). It is extremely common social problem at present. The fierce competition and irregular lifestyle places people under intense pressure and make them more vulnerable to fatigue. Patients with fatigue experience some depressive symptoms such as guilt, worthlessness, and suicidal ideation (Ahlberg et al., 2003). Furthermore, fatigue can seriously affect the quality of life and impair daily functioning.
Fatigue was found to be related to dysregulation of hypothalamic-pituitary-adrenocortical axis, polymorphism of neurotransmitters, alteration of autonomic system, decrease of tissue oxygenation, inflammation, and oxidative stress (Louati and Berenbaum, 2015). In particular, fatigue is common among individuals living with inflammatory diseases such as chronic fatigue syndrome (CFS), cancer, and autoimmune diseases (Louati and Berenbaum, 2015). The pro-inflammatory cytokine as a fatigue-associated factor was significantly related to the severity of fatigue (Cho et al., 2013). Bower has been reported that inflammatory cytokines act a vital role in physiological fatigue state (2014).
Macrophages, major inflammatory cells, produce interleukin (IL)-1β, IL-6, prostaglandin E2, nitric oxide (NO), and tumor necrosis factor (TNF)-α and when they are stimulated by lipopolysaccharide (LPS) or other pro-inflammatory cytokines (Vane et al., 1994). Uncontrolled production of these mediators can cause the pathogenesis of multiple chronic diseases including fatigue, inflammatory arthritis, asthma, endotoxic shock, cancer, and atherosclerosis (Cho et al., 2013; Galli et al., 2008; Lawrence et al., 2002). Inflammatory cytokines have been related to sleep disorders including sleep apnea syndrome, narcolepsy, idiopathic hypersomnia, and chronic fatigue syndrome (CFS) (Cho et al., 2013). Therefore, substances that inhibit the production of inflammatory mediators would be attractive to the invention of therapeutic agents or functional food for the treatment of fatigue.
Placenta is well-known as a specialized organ which is necessary for the developing fetus because it provides protein, vitamins, minerals and nutrient (Young and Benyshek, 2010). Moreover, placenta is a rich source of bioactive materials, such as cytokines, hormones, chemokines, and growth factors (Thomson, 2008). Previous studies demonstrated that placenta extract has numerous biological functions, including immune modulation, wound healing, anti-aging, post-partum depression, and cellular regeneration (Datta and Bhattacharyya, 2004;Hong et al., 2002; Kim et al., 2003). In addition, swine placenta extract has been shown to improve physical fatigue and liverfunction in humans (Kim et al., 2003; Mitsui et al., 2015). Previous studies have been reported that porcine placenta extract has anti-fatigue and anti-menopausal effects (Han et al., 2013; Han et al., 2015).
Fermentationis known to changeformation of active components and increases health benefits (Su et al., 2008). Fermented porcine placenta (FPP) is rich in leucine (Leu) and lysine (Lys) than porcine placenta, and result from previous study have indicated that it is nontoxic and safe (Mitsui et al., 2015). However, comparatively little is known of its biological effects or the detailed mechanisms involved. Thus, we investigated the anti-fatigue effect of FPP and major components, Leu and Lys on lipopolysaccharide (LPS)-stimulated RAW264.7 cells and forced swimming test (FST), which is a rodent behavioral test used for evaluation of anti-fatigue drugs or functional foods.
LPS, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),dimethyl sulfoxide (DMSO), and sodium nitrite were purchased from Sigma Chemical Co. (St Louis, MO, USA). Murine recombinant (r)TNF-α, rIL-1β, and rIL-6, anti-mouse TNF-α, IL-1β,and IL-6, and biotinylated mouse TNF-α, IL-1β, and IL-6, and were purchased from R&D Systems (Minneapolis, Minnesota, USA). Antibodies of caspase-1, inducible nitric oxide synthase (iNOS), and GAPDH were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) containing L-arginine (84 mg/l), Hank’s balanced salt solution (HBSS), fetal bovine serum (FBS), and other tissue culture reagents were purchased from Gibco BRL (Grand Island, New York, USA). FPP (Fermented Placenta Extract Powder A (K))was purchased from HORUS co., Ltd. (Japan). FPP was dissolved in distilled water (DW) and prepared at a dose of 10 µg/ml according to previous report (Han et al., 2013). FPP contained Leu(3.02 %) and Lys (2.12 %) as major components. Leu and Lys were purchased from Sigma Chemical Co. and dissolved in DW.
RAW264.7 macrophages were grown in DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat inactivated FBS at 37℃, 5% CO2 and 95% humidity. FPP was diluted with DMEM containing 10% FBS. Cells were pretreated with FPP (1 and 10 μg/ml) for 1 h prior to LPS stimulation.
RAW264.7 macrophages (1× 105 cells/well) were pretreated with FPP (1 and 10 μg/ml), Leu (10 μg/ml), and Lys (10 μg/ml) for 1 h and then stimulated with LPS (10 µg/ml) for 24 h. The amounts of TNF-α, IL-1β,and IL-6 secreted from RAW264.7 macrophages were measured by a modified enzyme-linked immunosorbent assay (ELISA). The ELISA was devised by coating 96-well plates of murine monoclonal antibody with specificity for TNF-α, IL-1β,and IL-6. Before use and between subsequent steps in the assay, coated plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween-20. All reagents used in this assay were incubated for 2 h at 37℃. The rTNF-α, rIL-1β, and rIL-6 were diluted and used as a standard. Serial dilutions starting from 10 ng/ml were used to establish the standard curve. After 2h incubation at 37℃, the wells were washed and then each of 0.2 µg/ml of biotinylated anti-mouse TNF-α, IL-1β,and IL-6 were added and the plates were incubated at 37℃ for 2 h. After washing the wells, avidin-peroxidase was added and the plates were incubated for 30 min at 37℃. Wells were again washed and substrate solution was added. Color development was measured at 405 nm using an ELISA reader (Molecular Devices Corp., Sunnyvale, California, USA).
RAW264.7 macrophages (1× 105 cells/well) were cultured for 24 h with FPP (1 and 10 μg/ml), Leu (10 μg/ml), and Lys (10 μg/ml). Cell aliquots were incubated with 20 µl of a MTT solution (5 mg/ml) for 4 h at 37℃ under 5% CO2 and 95% air. Consecutively, 250 µl of DMSO was added to extract the MTT formazan and the absorbance of each well was read using an ELISA reader at 540 nm (Molecular Devices Corp., Sunnyvale, California, USA).
RAW264.7 macrophages (1× 106 cells/well) were pretreated with FPP (1 and 10 μg/ml), Leu (10 μg/ml), and Lys (10 μg/ml) for 1 h and then stimulated with LPS (10 µg/ml) for 2 h. The enzymatic activity of caspase-1 was assayed using a colorimetric assay kit according to the manufacturer’s protocol.
RAW264.7 macrophages (1× 106 cells/well) were pretreated with FPP (1 and 10 μg/ml), Leu (10 μg/ml), and Lys (10 μg/ml) for 1 h and then stimulated with LPS (10 µg/ml). Whole cell lysates were made by boiling cell in sample buffer [62.5 mMTris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol, and 10% 2-mercaptoethanol]. Proteins in the cell lysates were then separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose paper. The membrane was then blocked with 6% bovine serum albumin in PBS and then incubated with antibodies. After washing in PBS-Tween-20 three times, the blot was incubated with a 1:3000 dilution of horseradish peroxidase-linked whole antibodies (Amersham Corp. Newark, NJ, USA). Antibody-specific proteins were visualized using the enhanced chemiluminesence detection system according to the recommended procedure (Amersham Corp. Newark, NJ, USA).
>
Measurement of nitrite concentration
RAW264.7 macrophages (1× 105 cells/well) were pretreated with FPP (1 and 10 μg/ml), Leu (10 μg/ml), and Lys (10 μg/ml) for 1 h and then stimulated with LPS (10 µg/ml) for 48 h. To measure nitrite, 100 µl aliquots were removed from conditioned medium and incubated with an equal volume of Griess reagent (1% sulfanilamide/ 0.1% N-(1-naphtyl)- ethylenediamine dihydrochloride/ 2.5% H3PO4) at room temperature for 10 min. The absorbance at 540 nm was determined using an ELISA reader (Molecular Devices Corp., Sunnyvale, California, USA). NO2‾ was determined by using sodium nitrite as a standard.
Male ICR mice (3 weeks old, 10 - 12 g) were purchased from the Dae-Han Experimental Animal Center (Daejon, Korea), and subsequently maintained at the College of Korean Medicine, Kyung Hee University. Experiments were initiated after 1 week to allow for adaption to the laboratory environment. Experimental procedures and animal care were approved by the Animal Care Committee of Kyung Hee University [KHUASP (SE)-15-080].After the first measurement of immobility times, the mice were divided into a DW (control), FPP (0.1, 1, and 10 mg/kg), Lys (10 mg/kg), and Leu (10 mg/kg) based on the recorded swimming times. DW, FPP (0.1, 1, and 10 mg/kg), Lys (10 mg/kg), and Leu (10 mg/kg) groups were orally administered to mice once per day for 21days. The FST proceed at the end of administration period for the 21days for 6min. The apparatus consisted of two Plexiglas cylinders (height: 25 cm, diameter: 10 cm) placed side by side in a Makrolon cage filled with water (10 cm height) at 23 - 25℃. Two mice were tested simultaneously for 6 min period inside the vertical Plexiglas cylinders; a nontransparent screen placed between the two cylinders prevented the mice from seeing each other. There were five mice in each group.After FST, mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/ kg) and xylazine (4 mg/kg). Blood was withdrawn from the hearts of forced swimming-tested mice into syringes. Then, the serum was prepared by centrifugation at 3000 rpm at 4℃ for 10 min. Spleen tissues were separated from mice and homogenized with homogenization buffer (20 mM HEPES pH 7.5, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1 M NaCl, 0.2 mM DTT). The protein extracts were prepared by centrifugation. The levels of inflammatory cytokines were determined by an ELISA reader. Cytokine levels in the spleen were divided according to the total protein levels, which were estimated using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA).
>
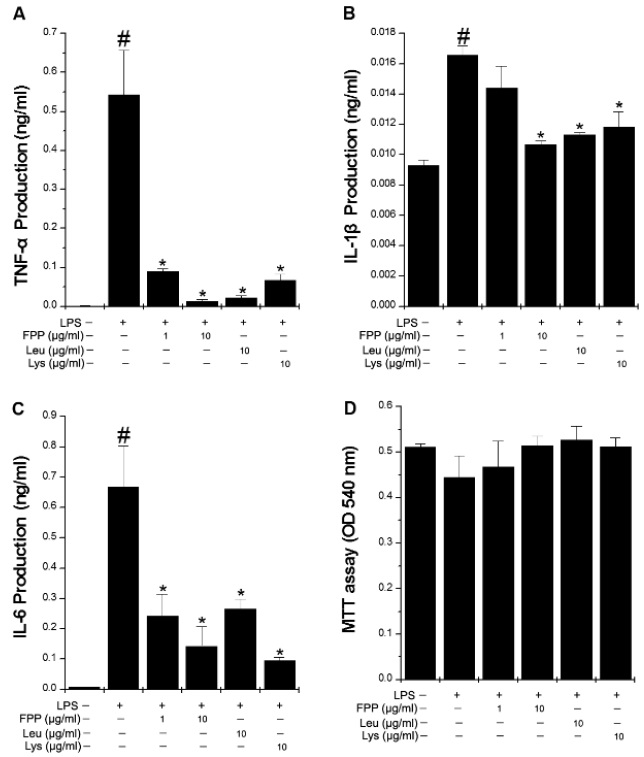
FPP suppresses LPS-induced inflammatory cytokine production in RAW264.7 macrophages
Inflammation has been related to CFS and inflammatory cytokines also play important roles in fatigue (Cho et al., 2013). In order to investigate whether FPP, Leu, or Lys can modulate LPS-induced inflammatory cytokine production, we performed the ELISA for inflammatory cytokine. As shown in Fig. 1A-C, LPS stimulation led to a strong increase in the productions of TNF-α, IL-1β, and IL-6, while FPP, Leu, or Lys significantly inhibited the LPS-induced TNF-α, IL-1β, and IL-6 production(
>
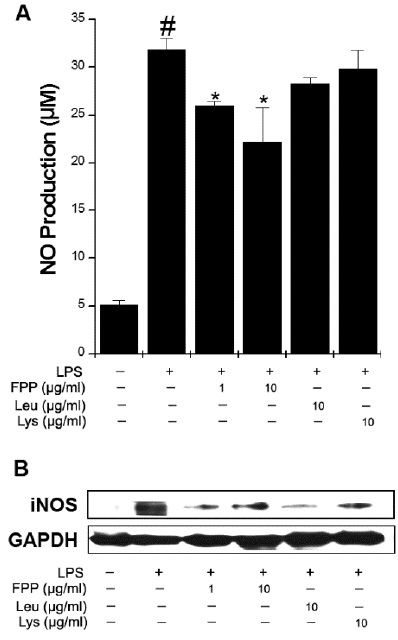
FPP suppresses LPS-induced NO productionand iNOS expression in RAW264.7 macrophages
NO plays vital roles in inflammatory responses. The levels of NO metabolites in CFS patients were higher than healthy control (Suárez et al., 2010). To determine whether FPP, Leu, and Lys can reduce LPS-induced NO production, we measured the NO using a Griess method. As shown in Fig. 2A, FPP significantly reduced the LPS-induced NO production(
>
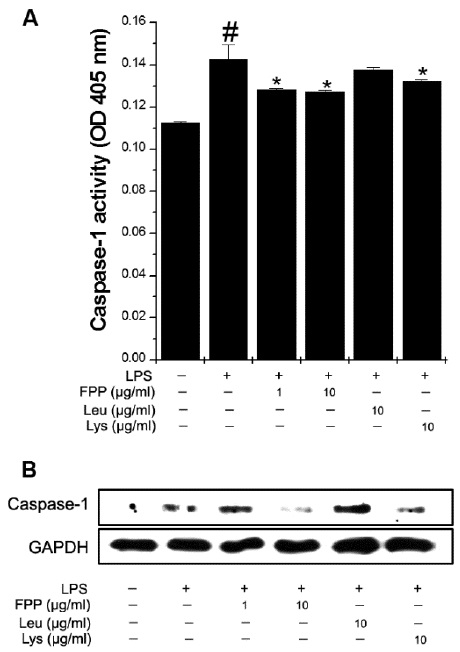
FPP suppresses LPS-induced caspase-1 activation in RAW264.7 macrophages
We next sought to determine how inflammatory cytokines are regulated by FPP, Leu, and Lys in activated RAW264.7 macrophages. Thus, we analyzed the caspase-1 activity because production of inflammatory cytokine was regulated by caspase-1. FPP and Lys significantly reduced the LPS-induced caspase-1 activity (Fig. 3A,
>
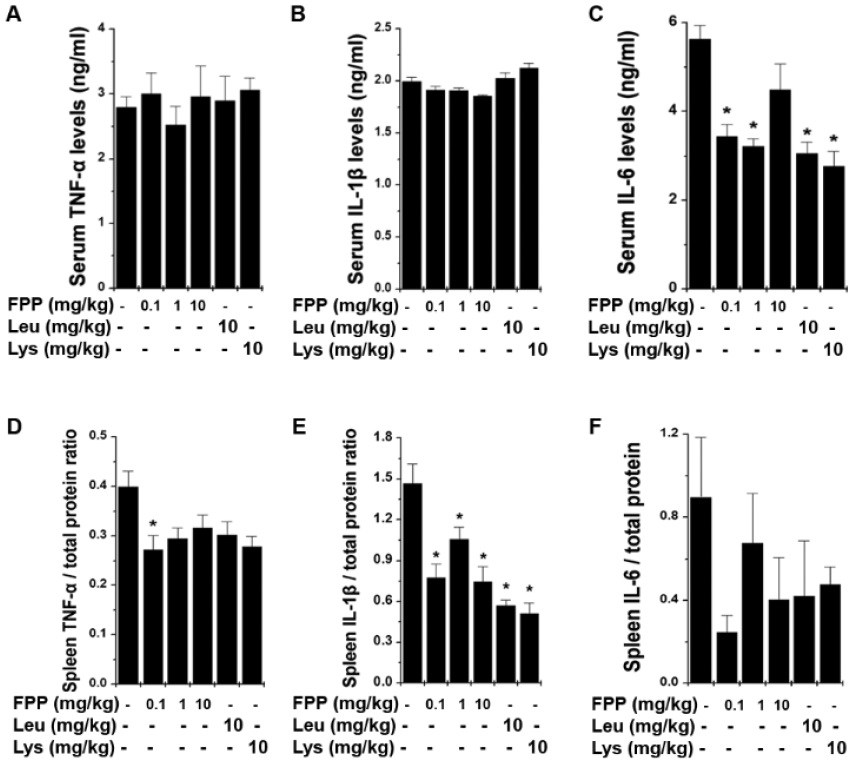
FPP suppresses inflammatory cytokine levels in FST fatigue animal model
To confirm whether FPP, Leu, and Lys could regulate the levels of inflammatory cytokine in fatigue animal model, we analyzed the inflammatory cytokine in the serum and spleen after FST. As shown in Fig. 4A-C, the protein levels of IL-6 were down-regulated upon administration of FPP, Leu, or Lys but not TNF-α and IL-1βin the serum (
Numerous studies have described that placental extract showed many biological functions such as anti-fatigue, anti-menopausal, immune modulation, wound healing, anti-aging, post-partum depression, and cellular regeneration effects (Datta and Bhattacharyya, 2004; Han et al., 2013; Han et al., 2015; Hong et al., 2002; Kim et al., 2003; Mitsui et al., 2015). In this study, we first reported that FPP, Leu, and Lys reduced the levels of inflammatory mediators through the inhibition of caspase-1 activation in RAW264.7 macrophages and FST animal model.
Fatigue is a common symptom in various inflammatory disorders including cancer and rheumatoid arthritis and it was correlated with high levels of peripheral inflammatory cytokines (TNF-α, IL-1, and IL-6), which generate fatigue or other behavioral symptoms (Bower, 2014). In patients with cancer, fatigue severity was correlated with levels of the inflammatory cytokines, IL-1 receptor antagonist (IL-1RA), TNF-α, and IL-6. In healthy individuals, IL-6 administration increases fatigue and sickness behavior and alters sleep structure (Späth-Schwalbe et al., 1998). Interestingly, alleviation of fatigue appeared in rheumatoid arthritis patients under treatment with biological agents targeting inflammatory cytokines (Rohleder et al., 2012). Anti-IL-6 (tocilizumab) and anti-TNF (certolizumab, infliximab, golimumab, etanercept, and adalimumab) have improved fatigue (Chauffier et al., 2012; Pollard et al., 2006; Scott, 1999). Fatigue is also associated with increased oxidative stress (Louati and Berenbaum, 2015). Previous study showed that elevated NO was considered as a possible mechanism for the fatigue (Suárez et al., 2010). NO is synthesized by iNOS enzyme, which is responsible for the production of NO using NADPH and oxygen molecules (Xia and Triffitt, 2006). Furthermore, the levels of iNOS protein were higher in CFS patients than in healthy controls (Maes et al., 2007). During the inflammatory response, activated macrophages induce the production and expression of inflammatory mediators, such as TNF-α, IL-β, IL-6, NO, and iNOS (Xia and Trffitt, 2006). The excessive inflammatory cytokine production is responsible for the initiation and regression of inflammatory process. In this study, we showed that FPP reduced the levels of inflammatory mediators on activated RAW264.7 macrophages and FST animal model. Therefore, we suggest that anti-fatigue effect of FPP might be derived through the regulation of inflammatory mediators. In the present study, Leu and Lys also reduced the levels of inflammatory cytokines. Hence, these results indicate that Leu and Lys are active components of FPP.
Caspase-1, a member of the caspase family, plays a vital role in causing apoptosis and inflammation (Gordon et al., 1990). Caspase-1 is activated by inflammasomes (cytosolic protein complex). Caspase-1 activation results in inflammatory response by inducing the reflux of neutrophils and the secretion of pro-inflammatory cytokines (Faubel et al., 2007). Caspase-1 deficient mice exhibited decreased levels of IL-6 (Kuida et al., 1995). Fibromyalgia associated with a fatigue was increased by caspase-1 complex protein, inflammasome and these data suggested inflammasome inhibitor as a new therapeutic intervention for treatment of fibromyalgia (Cordero et al., 2014). Thus, we postulated that FPP mediates anti-fatigue effects at least partly through suppression of caspase-1 activation. In this study, we confirmed that FPP and Lys suppressed the caspase-1 activation induced by LPS. This result suggested that the anti-fatigue effect of FPP might be derived through the anti-inflammatory effect by regulation of caspase-1 activation.
In conclusion, fatigue is serious social problem triggered by overwork, stress, or physical or mental illness. However, the treatment of FPP, Leu, and Lys effectively inhibited the levels of fatigue-associated inflammatory cytokines on
The authors report no conflicts of interest.