Arctic microalgae thrive and support primary production in extremely cold environment. Three Arctic green microalgal strains collected from freshwater near Dasan Station in Ny-Alesund, Svalbard, Arctic, were analyzed to evaluate the optimal growth conditions and contents of fatty acids. The optimal growth temperature for KNF0022, KNF0024, and KNF0032 was between 4 and 8℃. Among the three microalgal strains, KNF0032 showed the maximal cell number of 1.6 × 107 cells mL-1 at 4℃. The contents of fatty acids in microalgae biomass of KNF0022, KNF0024, and KNF0032 cultured for 75 days were 37.34, 73.25, and 144.35 mg g-1 dry cell weight, respectively. The common fatty acid methyl esters (FAMEs) analyzed from Arctic green microalgae consisted of palmitic acid methyl ester (C16:0), 5,8,11-heptadecatrienoic acid methyl ester (C17:3), oleic acid methyl ester (C18:1), linoleic acid methyl ester (C18:2), and α-linolenic acid methyl ester (C18:3). KNF0022 had high levels of heptadecanoic acid methyl ester (26.58%) and heptadecatrienoic acid methyl ester (22.17% of the total FAMEs). In KNF0024 and KNF0032, more than 72.09% of the total FAMEs consisted of mono- and polyunsaturated fatty acids. Oleic acid methyl ester from KNF0032 was detected at a high level of 20.13% of the FAMEs. Arctic freshwater microalgae are able to increase the levels of polyunsaturated fatty acids under a wide range of growth temperatures and can also be used to produce valuable industrial materials.
The Arctic region encompasses environments that are cold and frozen in most seasons. Many microorganisms have modified their metabolism to survive such harsh conditions. Cold-acclimated microorganisms have attained physiological and ecological adaptations to cold environment by evolving proteins and membranes with unique characteristics. Especially, membranes of psychrophiles integrate specific components of lipids to maintain and increase fluidity of membranes and other properties required for transfer of substrates (Deming 2002).
Physiological characteristics of cellular membranes in psychrophilic microorganisms, in particular the content of lipids and fatty acids in psychrophilic microalgal strains, have been investigated for their industrial application (Teoh et al. 2004, 2013, Ahn et al. 2012). Arctic microalgae are valuable microorganisms capable of converting carbon sources to materials that can be used as animal feeds and other valuable bioactive products during photosynthesis (Singh et al. 2005, Walker et al. 2005, Chisti 2007). Among the many products from microalgae, biofuels including biodiesels and methane are renewable products of microalgae biomass and biohydrogen generated by photosynthesis (Fedorov et al. 2005, Kapdan and Kargi 2006, Spolaore et al. 2006). The application of oil crops such as soybean, corn, and palms is not feasible for full-scale biofuel production because of the requirement for massive areas of land and management of food security in less-developed countries (Chisti 2007, Vello et al. 2014). In contrast, microalgae can be a useful alternative that can help exceed the present production of fatty acids from food crops without affecting global demand for food (Vello et al. 2014). To date, many studies have investigated the development of microalgal biodiesel production (Hu et al. 2008, Schenk et al. 2008, Huang et al. 2010, Mata et al. 2010, Abomohra et al. 2013). Most microalgal strains produce various types of lipids (diglycerides and triglycerides) according to the culture conditions, growth stage, and different environmental stresses such as lack of inorganic nutrients and low temperature (Chisti 2007, Pandey et al. 2014). Under low concentration of inorganic phosphorus, the content and productivity of total lipids increased in freshwater
Microalgae can be commercially adapted to reduce the nutrients in wastewater, e.g., from cow farms and other feeding animals as well as from industrial factories (Mahapatra et al. 2013, Pandey et al. 2014). In addition, they have been used efficiently to remove the wastes and high amount of nutrients in conventional oxidation ponds and commercial algal ponds (Martínez et al. 2000, Zhang et al. 2008, Ruiz-Marin et al. 2010). Various types of nutrients in wastewaters can be effectively polished with high algal density and they increase the amount of lipids in green microalgae (Wang et al. 2010, Wang and Lan 2011). Therefore, continuous microalgal cultivation is a possible method to preserve the environment and neutralize carbon content.
In the present study, growth rate and lipid production were analyzed in the cultures of 170 microalgal strains collected in both polar regions and maintained in the Korea Polar Research Institute (KOPRI) Culture Collection for Polar Microorganisms (KCCPM). Three Arctic freshwater green microalgae were selected to study cold-acclimated characteristics and relatively high contents of lipids. We analyzed growth rate under various temperature regimes and lipid production at low temperature in these microalgal strains and showed that three Arctic microalgal strains exhibit increased growth and high content of polyunsaturated fatty acids in the cells at low temperature. Given these physiological characteristics, the three Arctic microalgae studied here can be valuable resources to generate important products such as bio-diesels.
>
Isolation of microalgal strains
Polar microalgal strains were collected near King Sejong Station in the Antarctic and Dasan Station in the Arctic. Most of the microalgal strains were isolated and maintained in the corresponding growth media for freshwater and seawater, according to the sampling site. Bold’s basal medium (BBM) and Tris-acetate-phosphate (TAP) medium were used as culture media for freshwater strains, and F/2 medium (Guillard and Ryther 1962) was used for seawater strains. To maintain the culture collections, all samples were incubated at 2-3℃ under continuous light from light-emitting diodes (35 μmol photons m-2 s-1). Each microalgal strain cultivated was classified following the systems of KOPRI Arctic (North) Freshwater (KNF) strain and number of strains was maintained sequentially
>
Identification of the Arctic microalgae KNF0022, KNF0024, and KNF0032
Morphological characteristics of isolated KNF0022, KNF0024, and KNF0032 were investigated under a light microscope (Axio Imager A2; Carl Zeiss Microscopy GmbH, Göttingen, Germany) with Nomarski differential interference optics. Images were taken using a camera (AxioCam HRc; Carl Zeiss, Göttingen, Germany), and the size of cells was calculated with an image analyzer (Axiovision SE64 Rel. 4.8; Carl Zeiss, Oberkochen, Germany). For molecular identification of the Arctic microalgal strains, genomic DNA was extracted by a DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA), and the nuclear small subunit ribosomal DNA was amplified with primers G01 and G07 (Saunders and Kraft 1994) and Ex-
>
Determination of optimal growth media and temperature
Optimal growth media and growth temperature for Arctic microalgal strains were determined by observation of cellular status and cell counting of the samples. Approximately 8 × 104 cells of each sample were inoculated into freshwater media (BBM and TAP) and seawater media (F/2) and incubated at 12℃ for 2 weeks to determine the optimal growth media. Simultaneously, cultures were incubated at 4, 8, 12, and 20℃ in BBM under continuous light from light-emitting diodes (35 μmol photon m-2 s-1), without horizontal shaking. Total number of cells was counted twice using hemocytometer under an optical microscope (Axio Imager A2; Carl Zeiss) every 7 days and averaged for each sample.
>
Qualification of fatty acids by Nile red staining
To detect lipid droplets qualitatively, microalgal samples were stained with Nile red reagent (1 μg mL-1, 9-diethylamino-5H-benzo[α]phenoxazine-5-one; SigmaAldrich, St. Louis, MO, USA) in acetone and incubated for 30 min at room temperature. The lipid bodies in stained cells were observed with fluorescence microscopy (Axio Imager A2; Carl Zeiss) and analyzed by AxioVision 4 software (Carl Zeiss Microscopy GmbH, Jena, Germany). The excitation and emission wavelengths for fluorescence determination were selected as 488 and 550 nm, respectively.
>
Fatty acid methyl ester (FAME) analysis
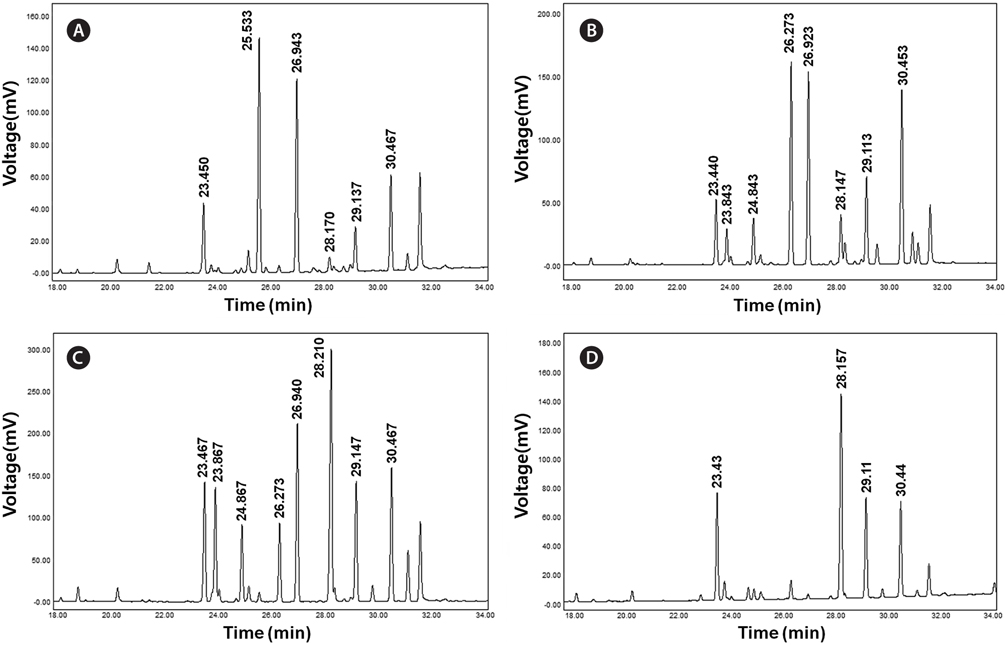
FAME analysis was performed in a gas-chromatograph (YL-6100GC; Young Lin Science, Anyang, Korea) with a flame ionized detector and HP-FFAP capillary column (30 m × 0.32 mm × 0.25 μm; Agilent Technologies, Santa Clara, CA, USA). Total lipids were extracted from 20 mg of freeze-dried sample according to the method described by Sasser (1990). Saponification was carried out with 2 mL of saponification reagent (7.5 M NaOH:CH3OH, 1 : 1 v/v) at 100℃ for 30 min. In the cooled samples, 4 mL of methylation reagent (CH3OH:6 N HCl, 1 : 1 v/v) was added and the samples were incubated at 80℃ for 10 min. After incubation, the reactions were incubated with 2.5 mL of extraction reagent (hexane:methyl tert-butyl ether, 1 : 1 v/v) for 10 min. The lower aqueous phases were removed by pipetting and approximately 6 mL of 0.5 M NaOH was added to the organic phase. The organic phases were loaded into a gas chromatography vials and components of fatty acids were analyzed. FAME analysis was performed with the following conditions: 1) constant flow mode at 3 mL min-1; 2) initial temperature at 100℃ for 5 min increased by 4℃ min-1 to 240℃ and held for 20 min; and 3) detector temperature of 250℃. Each of the FAME components was identified and quantified using a Supelco 37 Component FAME Mix (Sigma Aldrich). Components and molecular structures of each FAME from microalgal strains were identified by the TurboMass ver. 5.2.0. program (Perkin-Elmer Inc., Waltham, MA, USA). Total fatty acids were quantified against the internal standard (1 mg of C16:0 fatty acid in hexane) and the values were summed to express the unit as milligrams of FAMEs per gram of dry cell weight (DCW).
>
Cell morphology and phylogenetic relationships of KNF0022, KNF0024, and KNF0032
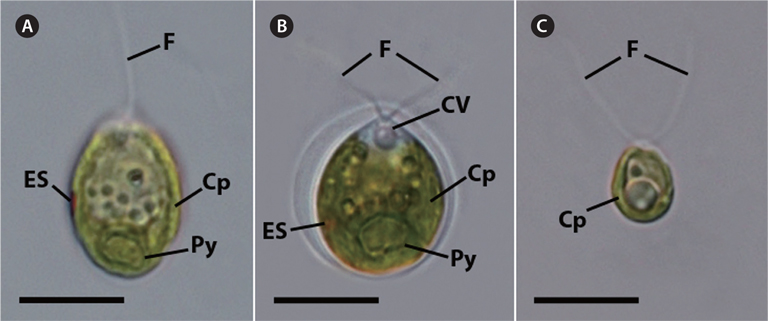
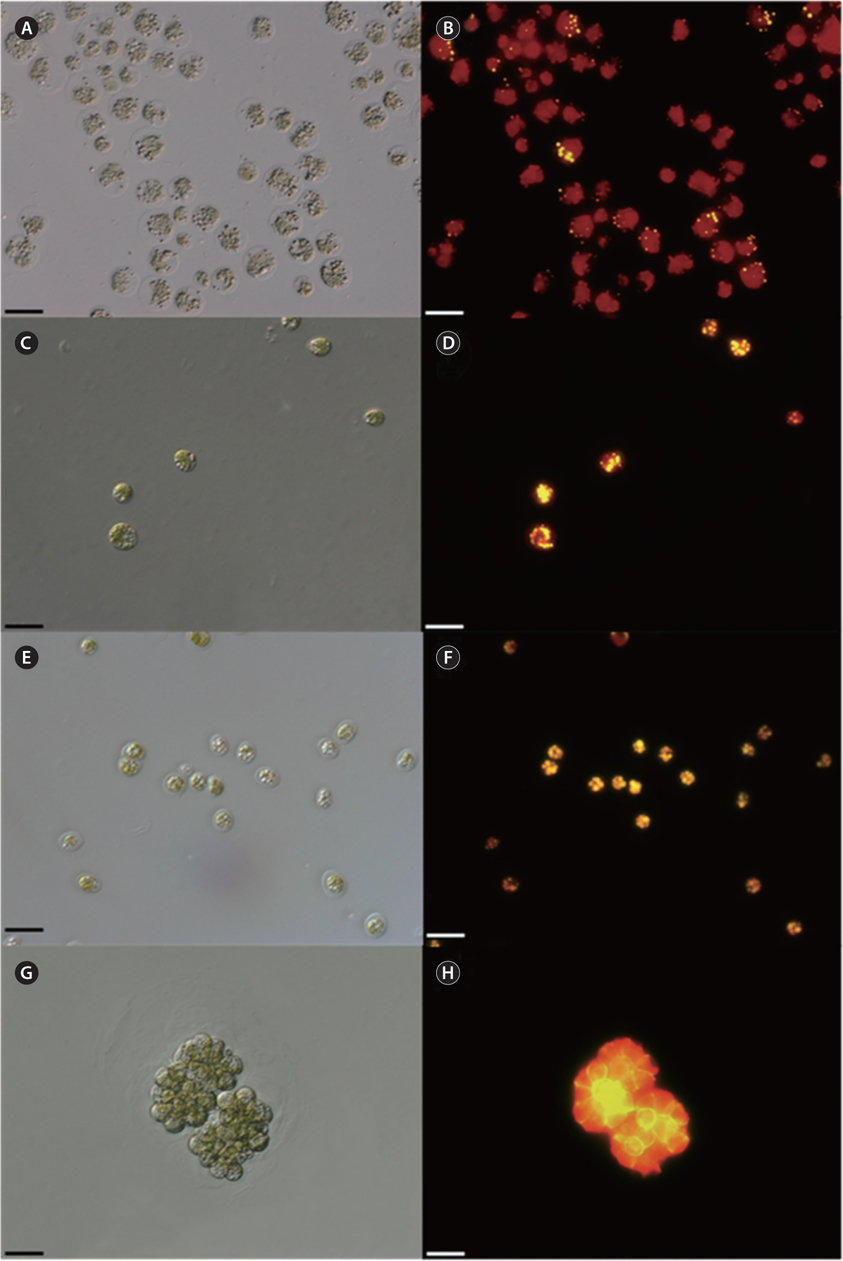
The vegetative cells of KNF0022 (Fig. 1A), KNF0024 (Fig. 1B), and KNF0032 (Fig. 1C) were solitary, biflagellate, and ovoid in shape; 15-22, 10-16, and 8-11 μm long; and 8-19, 10-15, and 5-7 μm wide, respectively. The cells had a single cup- or urn-shaped chloroplast (Fig. 1A-C), a prominent eyespot (Fig. 1A & B), a contractile vacuole (Fig. 1B), and a basal pyrenoid (Fig. 1A & B). These distinct morphological characteristics classified KNF0022 and KNF0024 in the
>
Determination of optimal growth media and temperature
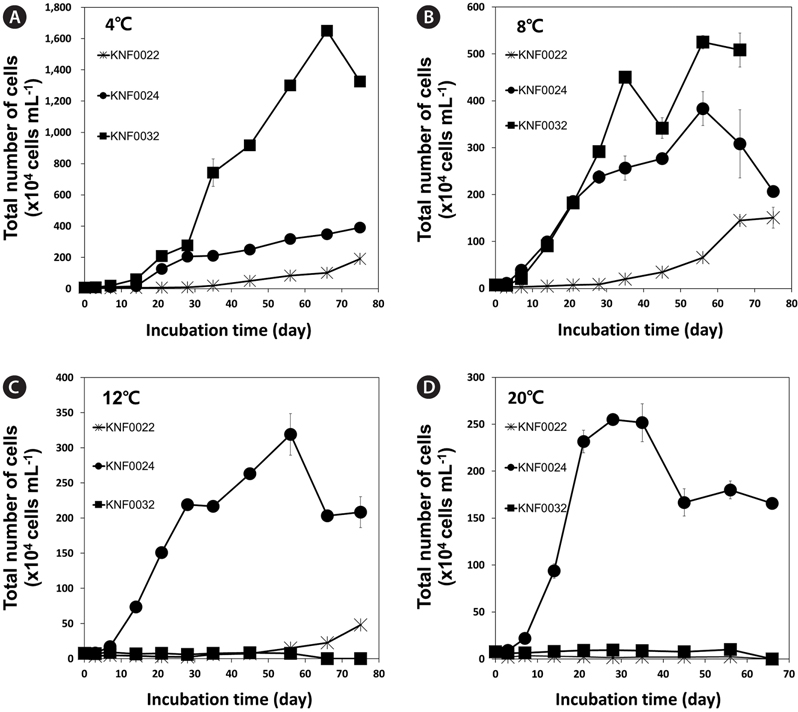
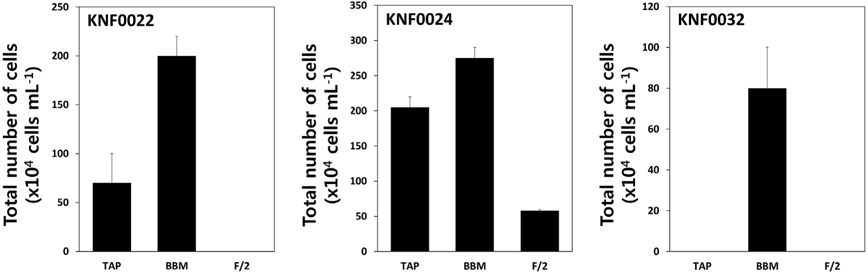
To determine the optimal culture media and growth temperatures, the growth rate of the strains was investigated under different temperatures and in different culture media. Psychrophilic microalgal strain KNF0032 had the maximal number of approximately 1,600 × 104 cells mL-1 at 4℃ (Fig. 3A). However, no visible growth of KNF0032 was detected at 12 and 20℃. The analysis of optimal growth media for the tested microalgal strains revealed that KNF0024 were capable of growing in all culture media (Fig. 4). All the samples gained less than 3 × 106 cells in 1 mL of BBM, but the cell number was higher than that in the TAP medium. KNF0032 was able to grow only in BBM (Fig. 4). The series of analysis of optimal growth media showed that all the Arctic microalgal samples tested in this study could be cultured in BBM and at relatively low growth temperatures.
>
Fatty acid production from Arctic microalgal strains
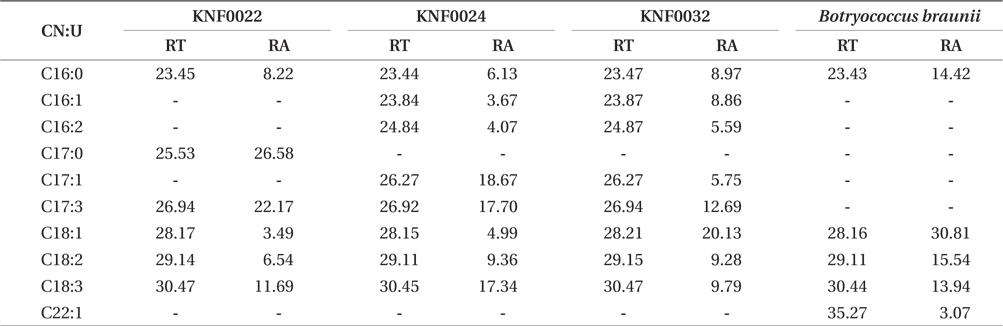
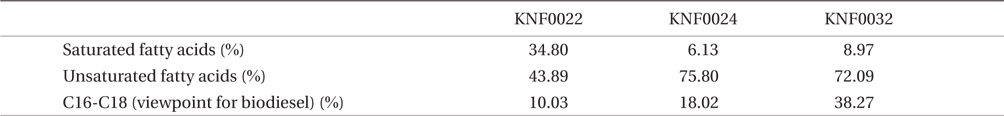
Concentration of lipid bodies accumulated in microalgal cells was determined using Nile red staining (Fig. 5). High concentration of lipid droplets was observed in Arctic microalgae as bright yellow and orange colors. In addition, lipids were present in abundance in KNF0032 cells (Fig. 5F). The composition and quantity of FAMEs in Arctic microalgae were analyzed with gas chromatography (Table 1, Appendix 1). In total, nine fatty acids from C16 to C18 were detected in Arctic microalgal strains. FAMEs in KNF0024 dominantly comprised cis-10-heptadecenoic acid methyl ester (C17:1), 5,8,11-heptadecatrienoic acid methyl ester (C17:3), and α-linolenic acid methyl ester (C18:3). Heptadecanoic acid (C17:0), a saturated fatty acid, was detected at a high level (26.58% of the total FAMEs) in KNF0022. Unsaturated fatty acids (palmitoleic acid and hexadecadienoic acid) from C16 comprised 7.74% and 14.45% of the total FAMEs in KNF0024 and KNF0032, respectively. In particular, oleic acid methyl ester in KNF0032 was detected in high levels (20.13% of the total FAMEs). Mono- and polyunsaturated fatty acids of C18 were present at 39.20% of the total FAMEs in psychrophilic KNF0032. The composition of saturated and unsaturated fatty acids in microalgal strains are summarized in Table 2. The contents of a series of FAMEs ranging from C16 to C18 were 78.69, 81.93, and 81.06% of the total FAMEs in KNF0022, KNF0024, and KNF0032, respectively. The productivity of fatty acids in KNF0022, KNF0024, and KNF0032 cultured for 75 days was 37.34, 73.25, and 144.35 mg g-1 DCW, respectively.
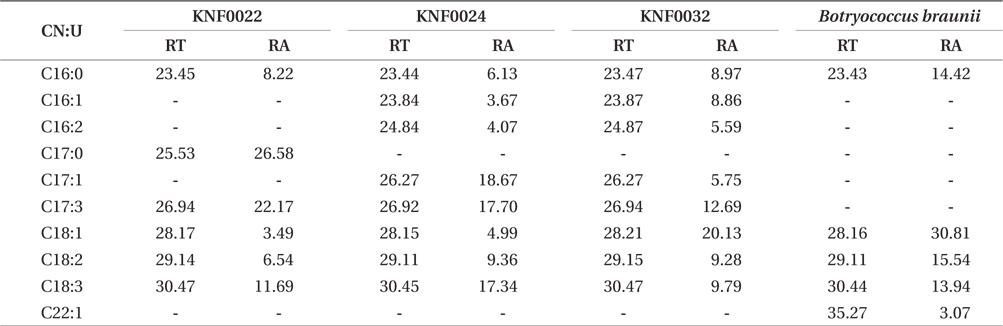
Composition of the surface percentage of fatty acid methyl esters with the retention time and relative area of the chromatographic peaks
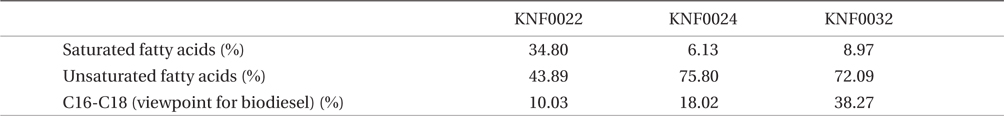
Summary of the composition of fatty acid methyl ester percentage for three Arctic green microalgae
Numerous molecular phylogenetic analyses have confirmed the polyphyletic nature of the genus
Microalgae are important 3rd-generation resources employed in the production of valuable by-products. Of 170 strains collected from the Arctic and Antarctic regions and maintained in the KCCPM, three Arctic microalgal candidates were selected based on their physiological traits such as growth rates under different temperatures and lipid body contents. All of the three Arctic microalgae grew in the temperature range from 4 to 8℃ (Fig. 3A & B). Among them, KNF0032 showed maximal growth and the highest number of cells at 4℃. Most of the microalgae found in Antarctica are eurythermal organisms that grow over a broad temperature range and, in addition, most of the isolates from the Victoria Land in Antarctica, including
To determine the optimum growth media for each microalgal strain, we chose three kinds of growth media: BBM, TAP, and F/2. The Arctic freshwater microalgae were tested whether they could survive and grow in the F/2 media, the seawater media. Most of freshwater microalgae are usually cultured in the BBM and TAP medium in laboratory conditions. All microalgal strains except KNF0032 were able to grow in the BBM and TAP medium (Fig. 4). Interestingly, psychrophilic KNF0032 strain grew only in the BBM, and KNF0024 was less sensitive to salinity as confirmed by the cellular growth of KNF0024 on the F/2 medium (Fig. 4). Previous studies have confirmed that
Qualitative analysis showed differing content of fatty acids in the microalgal strains (Fig. 5). Nile red staining has been commonly used as a rapid method for detection of lipid contents in various types of organisms. Most of the cells stained by Nile red contained lipid bodies observed as bright brown and orange colors (Fig. 5). Lipid content from the three Arctic microalgae was further characterized by gas chromatography (Appendix 1). The FAMEs of C16:0, C17:3, C18:1, C18:2, and C18:3 were commonly detected. The total unsaturated fatty acids (monounsaturated and polyunsaturated fatty acids) were dominant in over 70% of the strains (Table 2). In a recent study on the growth and fatty acid patterns in Arctic marine microalga ArM0029B (Ahn et al. 2012), C16:0, C18:1, and C18:2 were identified as dominant fatty acids, representing 39% of the total FAMEs; similar fatty acid content was observed for KNF0032 (38.38% of the total FAMEs) in the present study. Interestingly, only in KNF0022, up to 34.80% of total fatty acids were composed of palmitic acid (C16:0) and heptadecanoic acid (C17:0) (Table 1). In addition, KNF0032 had the highest level of oleic acid (20.13%), which was higher than that reported from the Antarctic
To date, most of the studies have focused on improving the amount of lipid bodies in intracellular compartments. In order to increase the content of fatty acids in microalgae, a variety of environmental stress factors including depletion of growth nutrients such as nitrogen and phosphorus have been applied (Chisti 2007, Nigam et al. 2011, Chaffin et al. 2012, Liang et al. 2013).