Carbon dioxide (CO2) is a component of the flue gas of power plants and automobile emissions. This gas is recognized as a primary greenhouse gas and is a presumed agent of climate change [1,2]. The drawbacks of the traditional MEA liquid method that is used for CO2 capture include the requirement for heavy equipment, and the toxic, flammable, corrosive, and volatile nature of the process [3]. Therefore, CO2 capture by means of adsorption in porous materials has received increasing attention because this method has proven to be superior than the conventional technologies in terms of the advantages associated with it. Compared to traditional processes, the convenient reversibility of adsorption on porous solid materials based on physisorption for the capture and release of CO2 makes this technique a greener and more cost-efficient method. To date, a variety of solid-based materials have been intensively studied for gas adsorption, especially CO2 capture, such as metal organic frameworks, covalent organic frameworks, zeolites, activated carbons, functionalized graphene, carbon molecular sieves, chemically modified mesoporous materials, etc [4-13]. Furthermore, the low concentration of CO2 in flue gas (ca. 15%) requires selective separation from the large volume of other component gases, mainly N2 [14]. Ideally, solid sorbents designed for CO2 capture should offer reduced energy consumption for regeneration, greater capture capacity, stability, selectivity, ease of handling, reduced environmental impact, etc. Currently, there is significant interest in the development of solid carbon adsorbents that are capable of selectively adsorbing CO2, because of their large surface area, porosity, abundance, cost efficiency, low density, fast adsorption kinetics, and high chemical and thermal stability [15-18]. Furthermore, these materials offer some advantages in terms of ease of handing, pore structure, and surface characteristics, as well as low-regeneration energy [19]. Therefore, carbon materials are currently considered attractive candidate sorbents for CO2 capture in the development of alternate clean and sustainable energy technologies. Based on these strengths, increasing efforts have recently been devoted to the synthesis of element-doped porous carbons that combine the high porosity and unique properties of doped carbon frameworks. The MCM-48 templated carbons have a high porosity without activation usually required to develop an accessible porous structure. Nitrogen doping has earned particular distinction because of the enhanced surface polarity, electrical conductivity, and electron-donor tendency conferred to the mesoporous carbons by nitrogen incorporation, which enables their application in CO2 capture, electric double-layer capacitors, fuel cells, catalysis, etc [20,21]. In the present study, we describe an alternate approach for the production of nitrogen-containing carbon materials with a cubic structure that facilitates CO2 diffusion and capture.
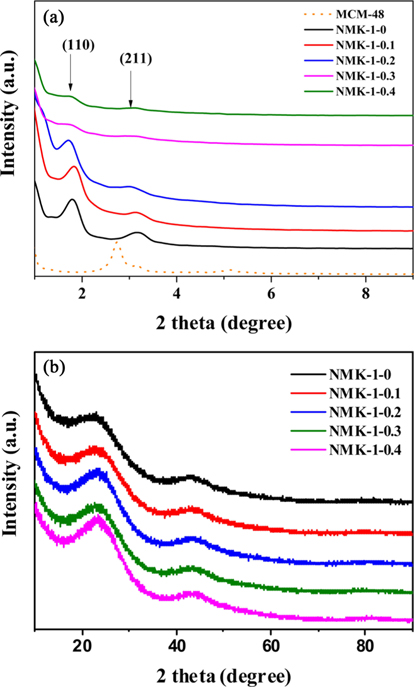
Fig. 1a presents the low angle X-ray diffraction (XRD) pattern of MCM-48.The pattern exhibits several well-resolved peaks in the low-angle range of 2
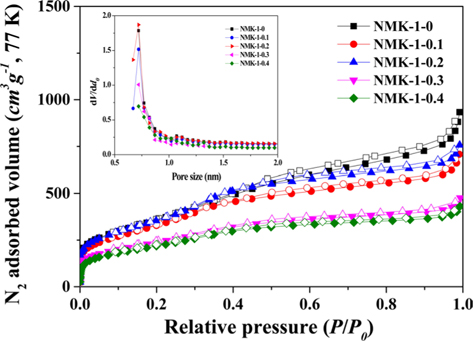
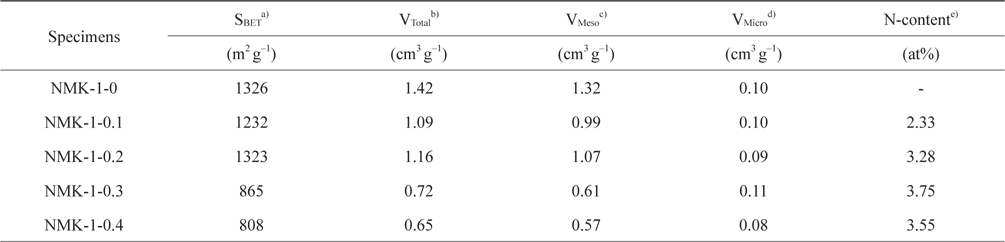
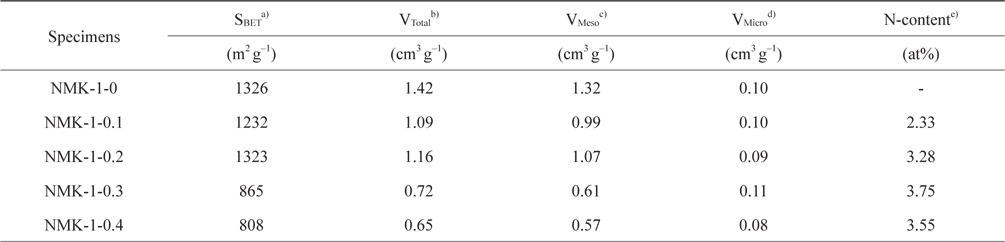
Fig. 3 shows the nitrogen isotherms of the porous carbon matrixes obtained from the mesoporous silica MCM-48 template using pyrrole, as described above. The NMK-1-0 sample had a large specific surface area (about 1330 m2 g–1) and large primary pore volume (1.32 cm3 g–1) (Table 1). Therefore, the nitrogen specific surface areas provided here are roughly estimated and the actual specific surface areas are probably much closer to that reported before (about 1300 m2 g–1) on the basis of a nitrogen BrunauerEmmett-Teller (BET) analysis. Further structural information was obtained by comparing the pore diameter of NMK with that of the silica wall thickness in MCM-48. The nitrogen adsorption/desorption isotherm and the corresponding pore size distribution for NMK-1-0 showed an inflection in the desorption curve, characteristic of capillary condensation within pores 3–4 nm in diameter, as shown in Table 1 and Fig. 3. The results of these nitrogen adsorption/desorption experiments are consistent with the data from the transmission electron microscopy analysis.
Seventy-seven K/N2 adsorption/desorption isotherms and micropore size distributions of the NMK-1 carbon materials
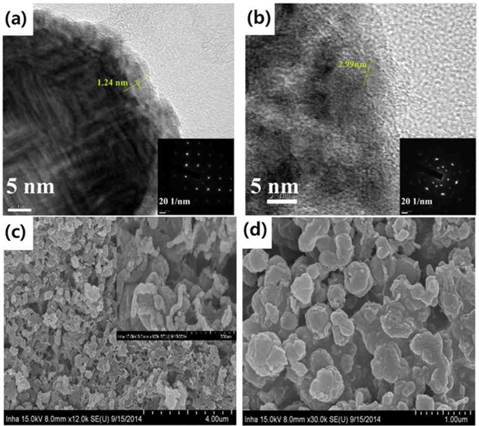
The pore diameter should be equal to the wall thickness of MCM-48 if the carbon synthesis simply followed a geometrical replication process. The silica wall thickness of MCM-48 was 1.24 nm, as determined from a TEM analysis of MCM-48 single crystals in Fig. 2a [29]. However, the pore diameter of the carbon replica was approximately twice the silica wall thickness in Fig. 2b. This difference demonstrated that structural transformation of the carbon frameworks took place upon removal of the silica wall. This structural transformation might be induced by strain in the carbon frameworks. A large contraction of the volume might also occur when pyrolysis of organic compounds to carbon takes place without external constraints. As shown in Fig. S3 micropores were present in the pore size distribution of all synthesized NMK-1-x carbons, where the pores created by the replicating process with diameters of about 0.72 nm were expected to facilitate diffusion of CO2 inside the pore channels for effectively capturing CO2.
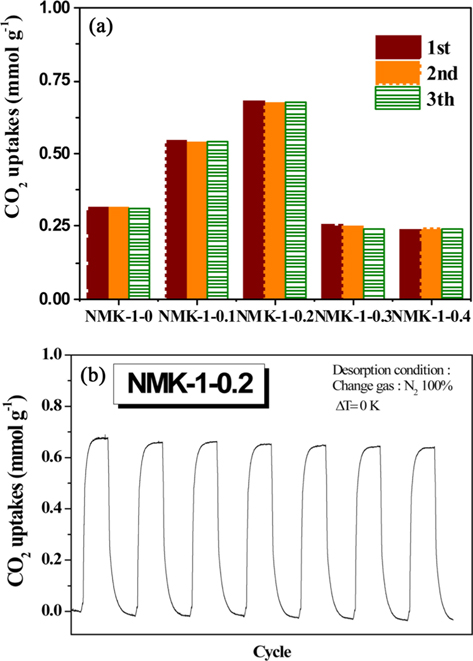
Notably, a very intense, sharp peak at approximately 2.7 nm was observed(Fig. S3), indicating the formation of a highly uniform structure in the NMK-1-0.2 sample. The pore structure of NMK-1-0.2 seemed to be enhanced by the presence of the pyrrole reagent, and mesopores and micropores dominated the distribution profile of this sample compared to other samples. The CO2 adsorption capacity of the porous carbons was investigated at 333.15 K (60℃) under flue gas conditions [30]. A comparative analysis of the CO2 adsorption ability of the NMK samples is presented in Fig. 4a. The CO2 capture performance of the samples, evaluated
In contrast, the specific surface area and the porosity of NMK-1-x decreased notably when the pyrrole used in the synthesis process exceeded 0.3 g. To explain this trend, it was hypothesized that the observed degradation of the structural properties is a consequence of pyrrole polymerization outside of the MCM-48 pores [31]. Generally, the porosity of the resulting carbon materials is strongly dependent on the degree of template pore filling. After infiltration into the template pores, the carbon precursor undergoes polymerization. However, release of the carbon source from inside the template pores also occurs during polymerization. Deposition of the released precursor (sucrose and pyrrole) on the external template surface leads to non-porous carbon and consequently reduces the porosity of the carbon materials. Therefore, the CO2 capture ability of NMK-1- 0.3 and NMK-1-0.4 is two times less than that of NMK-1-0.2, even though the nitrogen deposited on the surfaces of the former is higher than that on the latter. Moreover, it is proposed that the nitrogen is mainly anchored on the external interface, resulting in lower CO2 performance of these samples compared to NMK-1-0.2 and NMK-1-0.1 where the nitrogen functional groups are located in the pores. Regeneration of NMK-1-0.2, having the highest CO2 sorption, was examined using a TGA Pyris (Perkin Elmer, Waltham, MA, USA) 1 instrument. Gas-cycling experiments were conducted at 60℃ under dry conditions: 15% CO2 in pure N2, and pure N2 for the desorption process at 100℃, respectively. The regeneration experiment indicated no significant decrease in the CO2 adsorption capacity of NMK-1-0.2 after 8 cycles under these testing conditions in Fig. 4b. This indicates that NMK-1-0.2 exhibits high-capacity separation, requiring mild conditions for regeneration.
In this work, NMK-1-0.2 exhibits excellent CO2 adsorption capacity of 0.68 mmol g–1 at 333.15 K (1 bar, 15% CO2) and good regeneration performance. Thus, it is a promising adsorbent for energy-efficient CO2 capture from post-combustion processes.
Mesoporous siliceous materials were prepared at room temperature following the typical procedure. First, 1.2 g of cetyltrimethylammonium bromide (CTAB) was dissolved in water (50 mL) and stirred at room temperature (30℃) until all the surfactant molecules had dissolved. After adding 25 mL of ethanol, the solution was stirred for 5 min at 30℃. A 6 mL liquid portion of aq. NH3 (0.09 mol) was added, and the solution was stirred vigorously. Finally, 1.8 mL (8 mmol) of the silica precursor, tetraethyl orthosilicate (TEOS), was added and the mixture was stirred at 300 rpm for a further 4 h. The typical composition of the initial gel is 0.41 CTAB: 11 aq. NH3: 1.0 TEOS: 53 ethanol: 344 H2O. The obtained precipitate was filtered off and washed with deionized water several times. The obtained white powder was dried at room temperature overnight. The dried powder was calcined in static air at 550℃ at a heating rate of 3℃ min–1 and maintained at 550℃ for 9 h to remove the surfactant molecules.
The nitrogen-doped mesoporous carbons are denoted as NMK-1. Carbonization experiments were performed with sucrose, sulfuric acid, and various amounts of pyrrole. Typically, the synthesis of NMK-1-x involved dissolution of 1.25 g sucrose, 0.2–1.2 g pyrrole, and 0.14 g H2SO4 in 5.0 g H2O; this solution was then combined with 1 g MCM-48 [19,22]. The sucrose solution corresponded approximately to the maximum amount of sucrose and sulfuric acid that could be contained in the pores of 1 g MCM-48. The resultant mixture was dried in an oven at 100℃, and the oven temperature was subsequently increased to 160℃. After 6 h at 160℃, the MCM-48 silica containing the partially carbonized organic masses was added to an aqueous solution of 0.75 g sucrose, 0.08 g H2SO4, and 5.0 g H2O. The resultant mixture was again dried at 100℃, and the oven temperature was subsequently increased to 160℃. The ultimately obtained black powder was heated to 800℃ in an inert nitrogen atmosphere at a rate of 5℃/min, and the oven was maintained at this temperature for 2 h. The carbon-silica composite thus obtained was washed with a 1 M NaOH solution in 50% ethanol-50% H2O twice at 90℃ in order to dissolve the silica template completely. Removal of the silica template was also performed with HF solutions instead of NaOH. The carbon samples obtained after silica removal were filtered, washed with ethanol, and dried at 120℃. The synthesized samples were denoted as NMK-1-x, where x corresponds to the weight of pyrrole added in the reaction (from 0 to 0.4 g).
XRD analyses were carried out on a powder diffractometer (D2 PHASER, Bruker, Karlsruhe, Germany) with CuKα radiation (λ = 1.5406 Å). The textural properties of the synthesized samples were investigated using a gas adsorption analyzer (Belsorp-max, BEL Co., Japan). N2 adsorption/desorption isotherms were measured at 77 K in liquid nitrogen baths. The specific surface areas and porous volumes were determined using the BET equation, as well as the Horvath-Kawazoe and Barrett-Joyner-Halenda methods.
A TGA was performed to evaluate the CO2 adsorption performance under flue gas conditions, using a Pyris 1 TGA (Perkin Elmer) [33]. All gases were of 99.99% purity. All samples were outgassed for 5 h at 200℃ under a N2 flow to remove guest molecules from the pore system, and kept at 60℃ for 20 min under a N2 flow before starting the CO2 adsorption measurement. The CO2 adsorption process was conducted for 60 min at 60℃ using 15% CO2 in N2 gas, and the desorption process was carried out at 100℃ over 60 min.