



Lignocellulosic biomass conversion to biofuels such as ethanol and other value-added bio-products including activated carbons has attracted much attention. The development of an efficient, cost-effective, and eco-friendly pretreatment process is a major challenge in lignocellulosic biomass to biofuel conversion. Although several modern pretreatment technologies have been introduced, few promising technologies have been reported. Microwave irradiation or microwave-assisted methods (physical and chemical) for pretreatment (disintegration) of biomass have been gaining popularity over the last few years owing to their high heating efficiency, lower energy requirements, and easy operation. Acid and alkali pretreatments assisted by microwave heating meanwhile have been widely used for different types of lignocellulosic biomass conversion. Additional advantages of microwave-based pretreatments include faster treatment time, selective processing, instantaneous control, and acceleration of the reaction rate. The present review provides insights into the current research and advantages of using microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to fermentable sugars in the process of cellulosic ethanol production.
Lignocellulosic biomass typically comprises agricultural residues such as corn stover, wheat straw, and rice straw; agricultural by-products such as hulls, bran, and bagasse; and energy crops such as switchgrass,
[Table 1.] Composition of lignocellulosic materials and wastes [2,3]
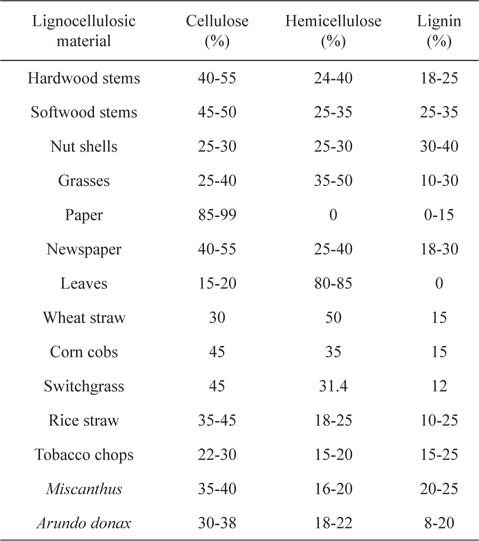
Composition of lignocellulosic materials and wastes [2,3]
2. Biomass-Derived Activated Carbon & Graphene
Activated carbon is a carbonaceous material that is prepared by the carbonization/pyrolysis of high carbon content materials, such as wood, petroleum, coal, etc. In order to reduce environmental pollution and the production cost, the preparation of activated carbon using renewable and less expensive precursors has attracted the interest of researchers worldwide. The precursors of interest are primarily agricultural and industrial lignocellulosic byproducts and forest wastes, such as rice straw, coconut shell, rubber-seed shell, hazelnut shell, cotton stalks, sugar beet bagasse, sawdust, bamboo, oil palm fiber, waste apricot, and coconut husk. The conversion of locally, abundantly available solid biomass waste into activated carbon would lend greater economic value by providing an alternative to costly activated carbon. Because of the rich carbon content of lignin, lignocellulosic biomass is a better choice to be used as precursor for producing activated carbon [4]. Activated carbon derived from lignocellulosic biomass is widely used for waste water treatment, air pollution treatment, chemical processes, and adsorption of volatile organic compounds [5]. Activated carbon is a well-known adsorbent due to its unique and versatile properties, namely high surface area, microporous structure, and favorable pore size, which allow access of gases/liquids into the internal pore surface and a high degree of surface reactivity [6].
The preparation of activated carbon from lignocellulosic biomass generally involves two main steps. The first step is char production from biomass with a carbonization/pyrolysis process, wherein moisture and volatile compounds are removed from the biomass [7]. Carbonization is usually done by pyrolysis in an inert atmosphere at temperatures lower than 500℃ [8] From char, activated carbon can be produced using three different types of activation processes: physical (e.g. CO2, steam), chemical (e.g. KOH, ZnCl2, strong base, Cu(NO3)2, H3PO4) and physicochemical (e.g. NaOH/CO2, Ce/CO2, CaO/steam, metal nitrate/steam). The activation process leads to the production of activated carbon with high porosity, large surface area, and high pore volume [6].
Graphene is the name given to a two-dimensional sheet of sp2-hybridized carbon. Porous graphene-like nanosheets with a large surface area were synthesized via a simultaneous activation-graphitization route from renewable biomass waste coconut shell [9]. Parshetti et al. [10] demonstrated a novel method to produce porous graphene-like nanosheets from lignocellulosic fiber of oil palm empty fruit bunches (EFB) by a thermal graphitization technique for CO2 capture. Recently, a simple, eco-friendly, and scalable method of obtaining graphene from dead camphor leaves (
3. Biomass-to-Fuel Conversion via Hydrolysis/Fermentation Pathway
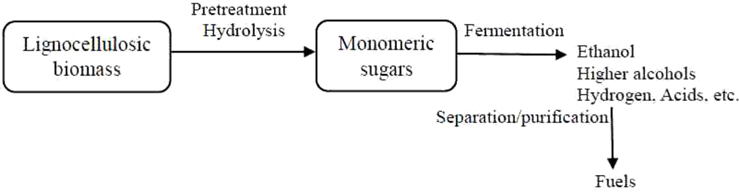
Lignocellulosic materials have strong potential to be used as raw materials for the fermentative production of biofuels. Bioethanol from lignocellulosic biomass (also called second-generation ethanol) shows some distinct advantages compared to first-generation ethanol as lignocellulosic biomass does not compete directly with crops grown for food, and therefore land use efficiency can be increased. In addition, the manufacture of chemicals and production of energy from biomass on a large-scale basis could be possible due to its abundant availability [12]. Biomass-to-fuel conversion is accomplished through different steps, namely pretreatment, hydrolysis, fermentation, and separation/distillation, as shown in Fig. 2.
Pretreatment of lignocellulose is a key step for efficient conversion of biomass to fuels. It affects the efficiency and methodology of the subsequent saccharification process [12]. The primary goal of pretreatment is to overcome the recalcitrant nature of cellulosic biomass [13]; an example of disruption of the higher order structure of lignocellulose upon pretreatment is depicted in Fig. 3 [2,14]. The pretreatment step is also aimed at increasing the surface area and pore volume, as well as reducing cellulose crystallinity. The recalcitrant nature of lignocellulosic biomass severely impedes the yield of fermentable sugars during hydrolysis. Biomass recalcitrance is due to two main physical factors, the crystalline structure of cellulose and matrix polysaccharides and the lignin coat of the cellulose fibril, which acts as a physical barrier to enzymatic attack on cellulose [15]. Chemical effects of pretreatment include increased solubility and depolymerization of lignocellulose polymer structures, and breakage of crosslinking between macromolecules [16].
The pretreatments can be classified into physical [17] or physicochemical [18], chemical [19], and biological [20] methods or combinations thereof [21-23]. The effectiveness of each type of pretreatment is presented in Table 2 [24-27]. Each type has unique advantages and disadvantages. However, most of the available pretreatment methods fail to achieve the desired result (i.e. effective disruption of biomass structure) and suffer from relatively low sugar yields and severe reaction conditions (high temperature and/or high pressure).
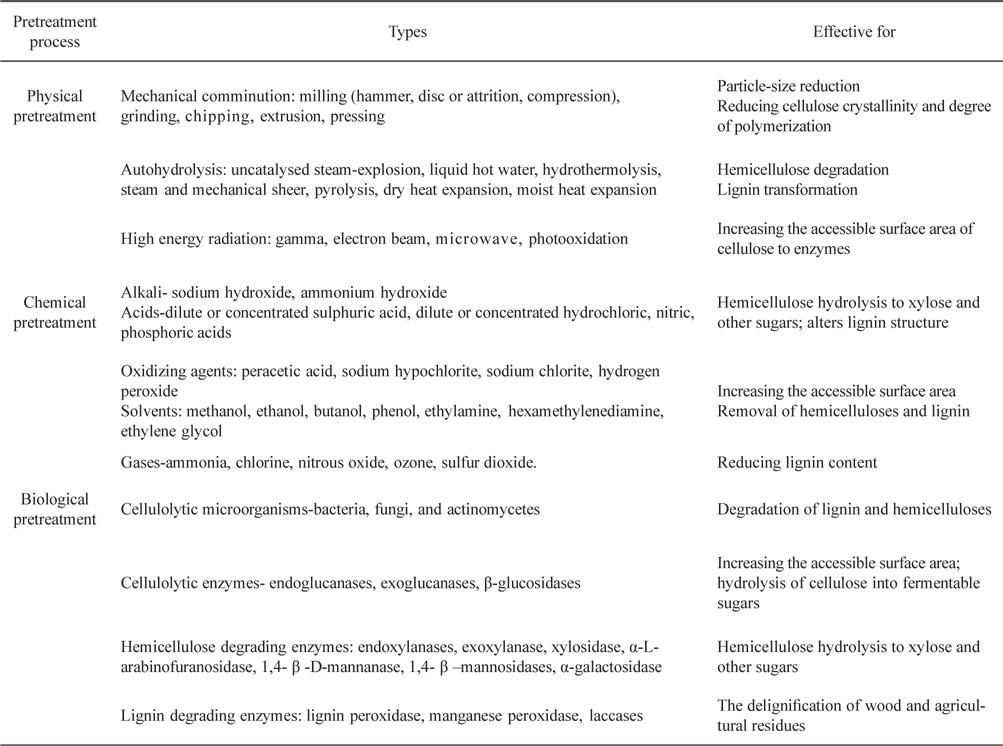
Various physical, chemical, and biological methods for the pretreatment of lignocellulosic biomass [24-27]
A pretreatment process featuring mild reaction conditions is highly desired because it would reduce sugar degradation and inhibitor formation and also lower initial capital costs [28]. The pretreatment step in biomass conversion is relatively expensive [29], and accounts for as much as 18% of the total ethanol production cost [1,30,31]. Enhancing yields through improved pretreatment would reduce all other unit costs. Therefore, the cost of production can be significantly reduced by increasing the pretreatment efficiency. At present, pretreatment processes are designed to facilitate the subsequent hydrolysis, thereby yielding maximum sugars with minimum fermentation inhibitory compounds. Pretreatment technologies are being investigated on both laboratory scale and in pilot plants all over the world.
3.1.1 Characteristics of an ideal pretreatment process
An ideal pretreatment should be affordable, with low energy and resource consumption, and should not produce any waste streams. It is necessary to promote high digestibility of cellulose, and must have high versatility for feedstock. In addition, there should be low sugar decomposition, low water or high solids, and low chemical consumption during the process. Furthermore, it should have low operational risk and must be safe to operate and the cost of construction materials should be low. It should further promote cost effective downstream processing.
4. Fundamentals of Microwave Heating Technology
Microwave heating involves a complex mechanism that depends on numerous factors i.e., propagation of microwaves governed by Maxwell’s equations for electromagnetic waves, the interactions between microwaves and dielectric properties of material, and the heat dissipation governed by heat and mass transfer. The electric field of commonly used irradiation frequency (2450 MHz) oscillates 4.9 × 109 times per second, and the sympathetic agitation of the molecules generates heat [32]. Therefore, dielectric properties govern the ability of materials to heat in microwave, including the dielectric constant (ε’) and dielectric loss (ε”). The ability of a material to convert electromagnetic energy into heat energy at a given frequency and temperature is calculated using:
where δ is the dissipation factor of the sample, ε” is the dielectric loss, which measures the efficiency with which heat is generated from the electromagnetic radiation, and ε’ is the dielectric constant, which gives the ability of a molecule to be polarized by an electric field [33]. A high value of the dissipation factor δ indicates large susceptibility to microwave energy. Microwave heating has the capacity to induce explosions among the particles and therefore improves the disruption of recalcitrant structures. Change in the ultra-structure of cellulose has been reported after irradiation [34].
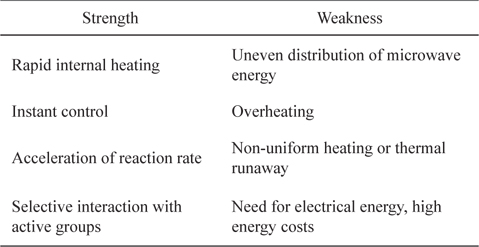
Microwave irradiation is highly effective and energy saving [35]. There are several advantages of microwave heating over conventional heating systems. These include reduced process energy requirements, selective processing, and precise control [36]. In addition, the heating is volumetric and rapid because the heat is generated internally through direct interaction between the electromagnetic field and components of the heated material [37]; i.e., microwaves couple directly with the molecules of the entire reaction mixture. In contrast, conventional heating is a slow and inefficient method of transferring energy into the reacting system. Some strengths and weaknesses of microwave heating are given in Table 3.
[Table 3.] Strengths and weaknesses of microwave heating for pretreatment process
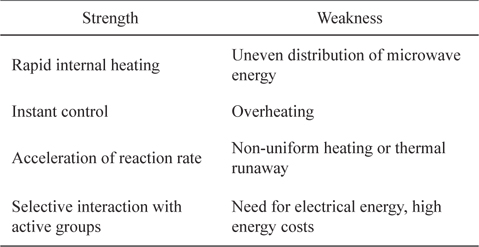
Strengths and weaknesses of microwave heating for pretreatment process
Microwave irradiation of biomass is a promising pretreatment process because it utilizes thermal and specific (non-thermal) effects generated by microwaves in aqueous environments [38]. Low temperature pretreatment is generally preferred to avoid hemicellulose degradation and to improve the pentose yield [39]. Due to its non-thermal and thermal effects, microwave radiation has successfully been applied for biomass pretreatment. These effects can cause fragmentation and swelling, leading to degradation of lignin and hemicellulose in biomass [40]. Microwave energy for biomass pretreatment was first introduced by Ooshima et al. [41], who used it to pretreat different hardwoods and softwoods.
5. Pretreatment Using Microwave Radiation
5.1. Microwave-assisted physical pretreatment
A novel, environment-friendly, and effective pretreatment for biomass was reported using combined ball milling and microwave irradiation [42]. It has been shown that combined ball milling for 1 h with microwave irradiation for 20 min can replace BM3 (ball milling for 3 h) and BM6 (ball milling for 6 h) with the same or higher glucose yield while saving 54.8% and 77.4% energy consumption, respectively. Furthermore, no chemicals are required for this process.
5.2. Microwave-assisted chemical pretreatment
A significant improvement in the total reducing sugar yield from rice straw and bagasse has been reported using microwave pretreatment [41]. In this process, rice straw and bagasse with water content of 84% or 94% were irradiated with 2450 MHz microwaves (170℃-200℃ for 5 min) in sealed glass vessels. This pretreatment resulted in enhanced substrate accessibility, 1.6 times greater in the rice straw and 3.2 times greater in the bagasse, for enzymatic hydrolysis compared to the untreated samples. Likewise, pretreated bagasse and rice hulls using microwave radiation yielded 77%-84% of total available reducing sugars [43]. Microwave pretreatment substantially improved the recovery of fermentable sugars from sugarcane bagasse [44]. Microwave-assisted dilute nitric acid pretreatment has been shown to enhance the enzymatic digestibility of rice straw by 14% when compared to the convection heating mode, and a reduction of the pretreatment time from 60 min to 7 min was also observed [45]. Microwave-assisted pretreatment (30 min at 700 W) of rice straw soaked in dilute alkali has resulted in an increase in glucose yield of 65% and total carbohydrate conversion of 79% compared with alkali-alone pretreatment [46].
Microwave-assisted alkali pretreatment has also been reported to be a potential alternative to wheat straw pretreatment for enzymatic hydrolysis [22]. In the aforementioned study, the wheat straw exhibited a weight loss of 48.4% and a composition of cellulose 79.6%, lignin 5.7%, and hemicellulose 7.8% after 25 min microwave-assisted alkali pretreatment at 700 W, compared with a weight loss of 44.7% and a composition of cellulose 73.5%, lignin 7.2%, and hemicellulose 11.2% after 60 min conventional alkali pretreatment. Greater amounts of lignin and hemicellulose were removed from wheat straw with microwave-assisted alkali pretreatment in shorter pretreatment time compared with the conventional alkali pretreatment. Production of ethanol at higher concentration and yield from microwave-assisted alkali pretreated wheat straw compared to conventional alkali pretreated wheat straw has been reported [47]. In this study, under simultaneous saccharification and fermentation (SSF), optimal conditions for the microwave-assisted alkali pretreated wheat straw were 100 g L−1 substrate, 40℃, 15 mg (cellulose) g−1 (substrate), initial pH 5.3, and 72 h. Under these conditions, the ethanol concentration reached 34.3 g L−1and the ethanol yield was 69.3%. The SSF optima for the conventional alkali pretreated wheat straw were 100 g L−1 substrate, 40℃, 20 mg (cellulase) g−1 (substrate), initial pH 5.3, and 96 h. Under the optimum conditions, the ethanol concentration and the ethanol yield were 31.1 g L−1 and 64.8%, respectively. In another study, the microwave pretreatment conditions were optimized for ethanol production from wheat straw [48]; under optimal pretreatment conditions, a ratio of biomass to liquid of 80 g kg−1, a NaOH concentration of 10 kg m−3, and microwave power of 1000 W for 15 min, the ethanol yields were 148.93 g kg−1 for treated and 26.78 g kg−1 for untreated straw.
Operating parameters that affect sugar recovery in microwave-assisted pretreatment of biomass recently have been discussed by Ethaib et al. [49]. They reported that biomass loading, microwave power level, and irradiation time are the important factors affecting microwave pretreatment efficiency, which reflects sugar recovery. A typical experimental setup for dilute acid pretreatment of biomass under a microwave irradiation environment is given in Fig. 4.
In a study on switchgrass, pretreatment using microwave-based alkali method yielded high sugar levels compared to conventional heating [50]. In a similar study, microwave-based alkali pretreatment of switchgrass and coastal bermudagrass resulted in better sugar yields during subsequent enzymatic hydrolysis; as high as 82% glucose and 63% xylose yields for switchgrass and 87% glucose and 59% xylose yields for Bermuda grass were reported [38].
Microwave-assisted alkali pretreatment (10% NaOH, 4:1 liquid-solid ratio, at temperature 90℃ and pretreatment time of 8 min) was shown to enhance the enzymatic digestibility of banana pseudostem; the total yield of reducing sugars reached 84%, compared to convectional heating mode of pretreatment (10% NaOH, 3:1 liquid-solid ratio, at temperature 90℃ and pretreatment time of 1.5 h), which yielded only 65% sugars [51]. Recently, microwave-assisted alkali pretreatment using 3% (w/v) NaOH at 180 W for 12 min was found to be effective in reducing lignin content (74%) and holocellulose (24.5%) from EFB of oil palm [52]. Subsequent enzymatic hydrolysis using microwave irradiation (microwave-assisted enzymatic reaction) at 100 W output power, temperature of 41℃-45℃, and incubation of 4 h was shown to have a profound influence on the yield of reducing sugar (411 mg g–1 of treated EFB biomass) compared to non-microwave enzymatic hydrolysis (178 mg g–1 of treated EFB biomass, after the incubation period of 48 h). Intanakul et al. [53] observed that microwave pretreatment (240 W, 10 min, at atmospheric pressure) enhances the removal of hemicellulose and lignin from cellulose in rice straw and bagasse. The key factor for separation was found to be high temperature (175℃-210℃) in the medium, which was achieved through microwave irradiation without a high pressure build up. Substantial reduction (up to 64%) of hemicellulose content and a moderate reduction (15%-17%) of lignin content were observed in oil palm trunk and oil palm frond lignocellulosic biomass after a microwave-alkali pretreatment method [54].
Microwave-assisted pretreatment of kenaf with 2% or 5% alkali (NaOH) was shown to be effective in delignification and decrystallization of fibers, and contributed to making the kenaf pulp more amenable to cellulase activity [55]. Microwave-assisted alkali irradiation has been reported to be an efficient pretreatment method to enhance cashew apple bagasse digestibility [56]. Tseng et al. [57] used the Taguchi method for statistical optimization of microwave-based heating of cellulosic biomass. This eight-order auto-regressive eXogeneous model helped to increase heating efficiency and reduce pretreatment time. Microwave-assisted KOH pretreatment (400 W for 30 min) significantly removed the recalcitrance of bamboo with low levels of fermentation inhibitors compared to acid pretreatment [58]. Selective delignifying capability of the H2O2-activated ammonium molybdate system energized by microwave radiation was reported [59]. In this study, a maximum sugar yield of 59.5% was reported by microwave irradiation of beech wood at 140℃ for 30 min, while external heating in an autoclave gave a sugar yield of 41.8%. The study also highlighted the effects of (peroxo) metal complexes as a microwave sensitizer and a delignifying agent for pretreatment of woody biomass.
Microwave irradiation was shown to enhance the dissolution of lignocellulosic materials in ionic liquids and subsequent fractionation of cellulose and lignin [60,61]. Microwave irradiation can facilitate the pretreatment of cellulose in ionic liquids [62].
5.3. Microwave-assisted two-stage pretreatment
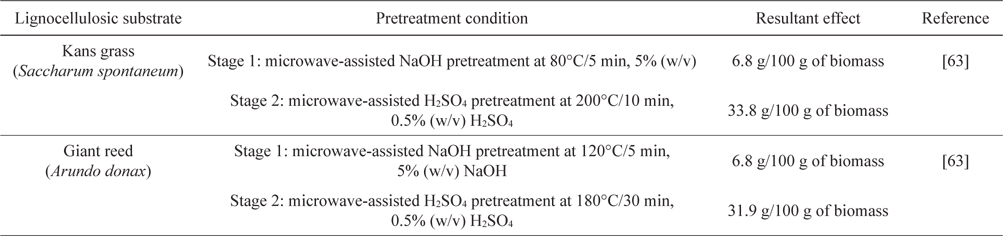
Recently, better monomeric sugar release was reported from Kans grass (
[Table 4.] Two-stage microwave pretreatment of Kans grass and Giant reed
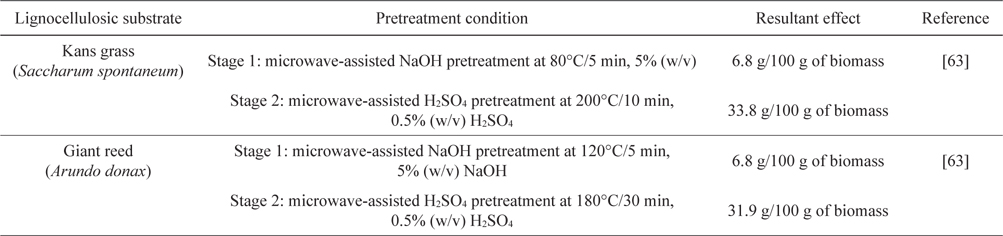
Two-stage microwave pretreatment of Kans grass and Giant reed
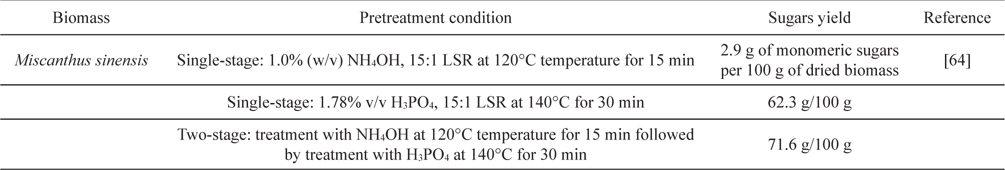
[Table 5.] Two-stage microwave pretreatment of Miscanthus
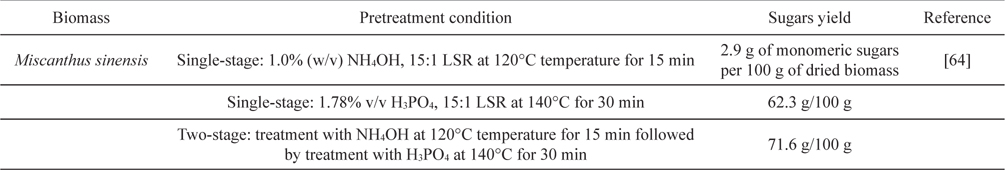
Two-stage microwave pretreatment of Miscanthus
Microwave-assisted NaOH pretreatment effectively removed approximately 85% of lignin content in Mission grass (
5.4. Microwave-assisted organosolvolysis
Organosolvolysis is a potential delignification process for the production of bioethanol from woody biomass. Microwave-assisted organosolvolysis in aqueous glycerol with organic and inorganic acids has been studied using Japanese cedar wood [66]. The pulp obtained by organosolvolysis with 0.1% hydrochloric acid (
Plasma sources are widely investigated as tools for biomass conversion. Atmospheric pressure, non-equilibrium plasmas have also been employed to treat different types of biomass in order to modify their surface and structure properties [67]. To separate the cellulose from the lignin-hemicellulose complex in biomass, plasma pretreatment (using flowing afterglow instead of direct exposure to the discharge plasma) has been applied. This results in increased contact surface and eventually improves the bioethanol production process [68-70]. For sugarcane bagasse pretreatment, a 2.45 GHz microwave plasma torch operating at atmospheric pressure in Ar-steam-air mixtures and in pure air plasmas was applied [71]; significant surface modification and destruction of the lignin layer due to plasma etching effect were observed. Zanini et al. [72] applied cold Ar plasma treatments in order to modify the structure of lignocellulosic fibers, and found that the chemical structure of lignin can be heavily modified by the plasma treatment. Microwave generated plasmas at atmospheric pressure have several advantages compared with those created by dc and RF electric fields: microwave discharges enable better energy transfer to the plasma, the microwave-plasma coupling efficiency is nearly 100%, power consumption is potentially lower, and allow throughput atmospheric processing is possible [73]. Recently, a microwave ‘tornado’-type air-water plasma source (Fig. 5) operating at 2.45 GHz and atmospheric pressure has been applied for pretreatment of cellulosic material [67]; the air-water highly reactive plasma environment provides a number of long-lived active species that are able to destroy the cellulosic wrapping. Oxygen containing reactive species such as O2 (a1 Δg) singlet delta molecules, OH radicals, HNO2, and NO2 have been shown to play important roles in the treatment process.
6. Effect on Subsequent Enzymatic Hydrolysis
Positive effects of microwave pretreatment on subsequent enzymatic hydrolysis have been reported. An increase in initial enzymatic hydrolysis rate of rice straw was observed after pretreatment by combining microwave irradiation with alkali; however, the hydrolysis yield remained unchanged [74]. Compared to other methods, microwave-assisted alkali pretreatment has been shown to be an efficient way to improve the enzymatic hydrolysis accessibility of corncob in a shorter amount of time and at a lower temperature; a total sugar concentration of 683.97 mg/g (or 45.60 g L–1) of pretreated corncob was obtained from the optimum pretreatment conditions of 2% NaOH at 100℃ for 30 min in a microwave oven [75]. Microwave-assisted KOH pretreatment yielded improvement of enzymatic hydrolysis of 8.7-fold (20.87%, glucose) and 20.5-fold (63.06%, xylose), respectively, at 48 h compared with untreated bamboo (2.41%, glucose; 2.94%, xylose) with a cellulase concentration of 15 FPU/g glucan [58]. Microwave-assisted dilute acid pretreatment has shown remarkable effects in promoting enzymatic saccharification of water hyacinth; the use of pretreatment conditions of 1% H2SO4 at 140℃ for 15 min followed by enzymatic hydrolysis yielded a maximum sugar reduction of 48.3 g/100 g, which accounts for 94.6% of the theoretical reduced sugar yield [76].
Drastic enhancement of the enzymatic hydrolysis of cellulose after microwave pretreatment of lignocellulosic material in ionic liquids was recently reported [77]. In this study, the cellulose saccharification percentage was approximately 20% for kenaf powders pretreated in choline formate, choline acetate, and choline propionate by conventional heating at 110℃ for 20 min. In contrast, approximately 60%-90% of cellulose was hydrolyzed to glucose after microwave pretreatment in the same ionic liquids at 110℃ for 20 min. The rate of enzymatic hydrolysis of cotton cellulose was increased 50-fold after ionic liquid dissolution pretreatment with microwave irradiation [78].
7. Conclusions and Recommendations
It can be concluded that emerging microwave-assisted pretreatment technologies are more efficient, rapid, and economically feasible for effective removal of hemicelluloses and lignin cover compared to their predecessors. A high capital investment is required for microwave equipment; however, the operating costs are lower compared to conventional heating methods or systems.
Adequate moisture in biomass feedstock is a key factor for successful biomass pretreatment using microwaves. In the case of woody biomass, steam-induced explosions inside the biomass structure due to high-powered microwave irradiation may increase the recalcitrance of the biomass as such explosions are usually accompanied by a rapid loss of moisture. Therefore, systems that can maintain a sufficient moisture level are recommended for superior pretreatment [79]. In addition, the use of pressurized reactors has been recommended since insignificant breakdown was observed under atmospheric pressure conditions, where temperatures of only +/−100℃ could be achieved.


![Composition of lignocellulosic materials and wastes [2,3]](http://oak.go.kr/repository/journal/20913/HGTSB6_2016_v17n1_1_t001.jpg)
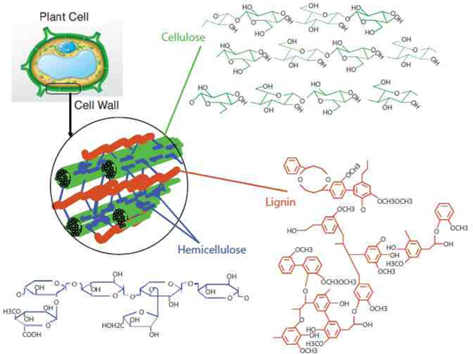
![Disruption of recalcitrant structures of lignocellulose upon pretreatment. Modified from Kumar et al. [2] and Hsu et al. [14].](http://oak.go.kr/repository/journal/20913/HGTSB6_2016_v17n1_1_f003.jpg)
![Various physical, chemical, and biological methods for the pretreatment of lignocellulosic biomass [24-27]](http://oak.go.kr/repository/journal/20913/HGTSB6_2016_v17n1_1_t002.jpg)

![Block diagram of a typical microwave-assisted dilute acid pretreatment of biomass (based on experimental setup by Chen et al. [40]).](http://oak.go.kr/repository/journal/20913/HGTSB6_2016_v17n1_1_f004.jpg)
![Block diagram of air-water ‘tornado’-type microwave plasmas for biomass treatment (based on experimental setup by Bundaleska et al. [67]).](http://oak.go.kr/repository/journal/20913/HGTSB6_2016_v17n1_1_f005.jpg)