Recently, porous carbon-based macrostructures consisting of low-dimensional carbon nanomaterials, such as one-dimensional carbon nanotubes and two-dimensional (2D) graphene sheets, have been intensively developed as next-generation pollutant sorbents that can replace the conventional absorbent materials [1-7]. The eminent absorption properties of these nano carbon-based sorbents (e.g., high loading capacities, rapid absorption rates, robust recyclability, and high selectivity) mainly stem from their extensive specific surface area (SSA) and intrinsic hydrophobicity [8,9]. In particular, three-dimensional (3D) graphene-based architectures constructed upon 2D graphene nanosheets offer great potential for efficient, ultralight, low-cost, environmentally benign, and mass-producible pollutant sorbents [3-5]. The promising features of the 3D graphene macrostructures, which include fast mass-transport kinetics, highly accessible surface area, and good chemical and mechanical stability, are based on a synergistic combination of internetworked macropores with the intriguing chemical, physical and textural properties of individual graphene sheets [3,4,6]. Three-dimensional macroscopic scaffolds assembled into highly macroporous N-doped graphene foams, prepared through a high-temperature process, demonstrated outstanding pollutant-absorbing properties [2]. However, this high-temperature process is undesirable for cost-effective mass-production because of its large consumption of energy and time. Besides, a too fast heating rate can seriously damage the microstructure of the resulting products. Alternatively, chemical and/or low-temperature thermal approaches have been employed to produce porous graphene-based materials on a large scale [3-6,10-12].
A systematic study of absorption parameters provides a design guideline of micro/macroscopic structures for advanced sorbents and thus, significantly contributes to optimizing their absorption performance. However, the sorption parameters are complicated by the physicochemical properties of absorbents and absorbates. These include the surface properties of absorbents (surface functional groups and hydrophobicity), textural properties of absorbents (from meso- to macropores and bulk densities), and physicochemical properties and chemical structures of absorbates (density, viscosity, and surface tension). Accordingly, the key parameters determining the absorption behavior of 3D graphene-based absorbents have not been fully specified.
Here, we demonstrate highly macroporous, slightly reduced graphene oxide (srGO) sorbents, and present results from a systematic investigation into the effects of specific parameters on their capabilities for absorption of oils and organic solvents. The 3D macroporous architectures were first assembled using an ice-templating process for graphene oxide (GO) dispersion and then, slight reduction as a function of incremental reduction time, produced 3D macroporous srGO-based sorbents with a light bulk density. A series of absorption parameters such as a SSA, a bulk density, hydrophobicity, and chemical structures were coupled together and thus, controlled by reduction time to understand the absorbing capability of 3D srGO-based sorbents. The parameters deterministic for the high absorption capacity (up to 319 times its own weight) were found to be the open porosity of the hydrophilic srGO materials, and a carbon-to-oxygen ratio (C/O ratio) slightly higher than ‘4’.
A GO dispersion with a concentration of 2 wt% was purchased from Angstron Materials Inc. (Dayton, OH, USA). The chemical reagents hypophosphorous acid (H3PO2) and iodine (I2) were obtained from Sigma-Aldrich Chemical Co. (Darmstadt, Germany). All chemicals were used without further purification.
2.2. Preparation of rGO-based sorbents
In order to prepare the 3D GO scaffolds, a GO dispersion with a concentration of 5 mg/mL (Angstron Materials Inc.) was immersed in a dry ice bath containing liquid nitrogen for 30 min to freeze the dispersion; then the ice was sublimated by freeze drying. The as-prepared GO scaffolds were placed in a pre-heated standard vacuum oven. After an oven vacuum of 1 × 10−2 Torr was achieved, the samples were reduced at the pre-heated temperature of 200℃ for 15 or 30 min, and then again for 1, 2, 3, 6, or 10 h.
The 3D macroporous srGO sorbents were produced
2.3. Characterization of the rGO macrostructures
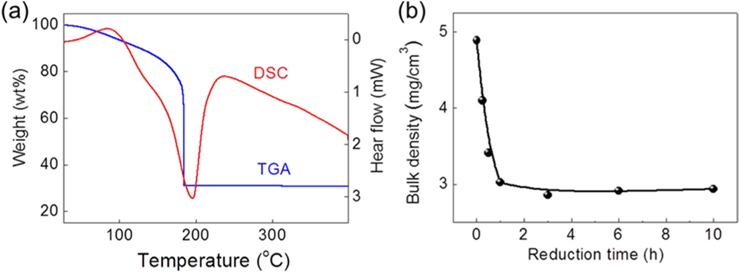
The morphologies of the rGO sorbents were observed using scanning electron microscope (LEO SUPRA 55; Carl Zeiss, Germany) and transmission electron microscopy (TEM; JEM-3011, JEOL, Japan). Their chemical compositions were analyzed using X-ray photoelectron spectrometry (XPS; AXIS Ultra DLD, Kratos, Inc., England)). Fourier transform infrared (FT-IR) spectra of all samples were recorded in the attenuated total reflectance (ATR) mode in the frequency range 4000–650 cm−1 on a Nicolet 6700 instrument (Thermo Scientific, USA). The spectrum was recorded as the average of 32 scans with the resolution of 8 cm−1. The textural properties of the rGO macrostructures were characterized by means of an N2 adsorption-desorption experiment using a surface area and pore size analyzer (BELSORP mini2, Japan). For this, the SSA and the pore size distribution could be determined from the linear part of the Brunauer-Emmet-Teller (BET) equation and the adsorption isotherm based on the Barrett-Joyner-Halenda (BHJ) method. The thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were conducted at 5℃/min in a temperature range of 25℃–1000℃ (TGA) and 25℃–400℃ (DSC) using a Dupont 2200 thermal analysis station.
Absorption capacities of the srGO-based sorbents were evaluated for a series of commercial oils and organic solvents, defined as;
where
3.1. Absorption capacities of 3D macroporous srGO-based sorbents
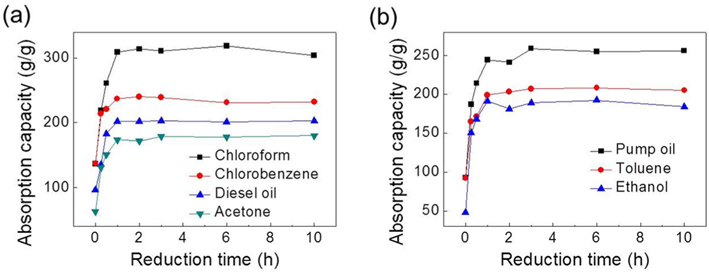
Fig. 1 displays the absorption capacities of samples as a function of reduction period from 0 h (i.e., GO) to 10 h. The absorption capacities of the 3D srGO-based sorbents were measured in a range from 319× (chloroform) to 179× (acetone) their own weights. Despite the low-temperature reduction process used here, these absorption capacities for oils and organic solvents are considerably higher than those in various carbon-based 3D architectures [1-3,5-7], except for the high-temperature-processed ultralight N-doped graphene frameworks [4]. Differently from the previous studies on the carbon-based sorbents with the maximum absorption values, the absorption capacities of our samples gradually increased with extension of reduction time up to 1 h, and thereafter became saturated at specific values. Such saturation of absorption capacities, which were confirmed using ten samples from different batches, indicates reproducible and reliable absorption performance in cryo-structured, low-temperature-processed macroscopic sorbents.
3.2. Surface chemistry of 3D macroporous srGO-based sorbents
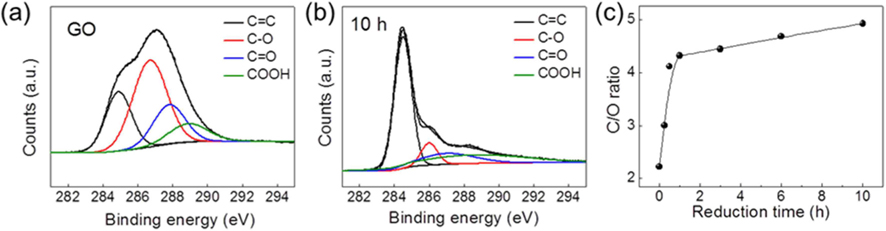
The freeze-casting of unreduced GO dispersion resulted in randomly oriented 3D networks framed with few-layered GO sheets. The resulting samples retained the inherent macroporous structure of 3D interconnected networks after thermal reduction for 10 h (Fig. S1). The degree of reduction of the GO, and variations in the chemical structures of samples during the reduction process, were characterized using XPS measurement. As shown in the C 1 s spectra (Fig. 2a), GO presents four carbon atomic components with a considerable degree of oxidation (
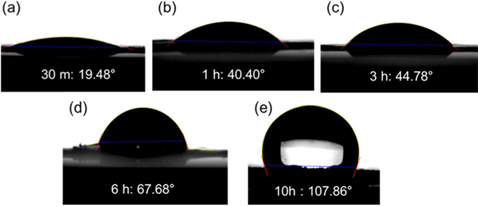
The degree of reduction described by the C/O ratio also influences the hydrophobicity of srGO-based macrostructures. The hydrophobicity is a parameter that influences the absorption capability for oils and organic solvents [2,21,22]. The hydrophobicity can originate from the combination effect of a bulk porous structure (macroscopic voids), micro and/or nano-textured surfaces (micro/nano-roughness and voids), and intrinsic hydrophobic surface properties [23]. Because the ice-templating approach produces highly macroporous structures pre-framed by the thin walls of few-layered GO sheets prior to their reduction, the differences in the hydrophobic properties of samples are associated with the textured surfaces and the hydrophobic surface property. The vacuum-assisted thermal reduction induces a wrinkling of the surface as well as decomposition of oxygen-containing groups on the basal plane in GO. This results in roughening of the surface texture to increase hydrophobicity, as observed from TEM images in Fig. 3 [11,18]. Nonetheless, the srGO sample thermally reduced for 6 h is still hydrophilic, as demonstrated by the water-drop contact angle of 67.68° in Fig. 4. By extending the heating time to 10 h, a hydrophobic surface with a contact angle of 107.86° could be obtained due to the decomposition of oxygen-containing groups. Here, it should be noted that the saturated absorption capacity of the hydrophilic srGO sample (thermally reduced for 1 h) could not be further enhanced, even though the samples were reduced for 10 h to achieve the hydrophobic surface. Accordingly, the absorption capacities of the hydrophilic srGO-based sorbents are comparable with those of their hydrophobic counterparts, as long as the residual water and surface hydroxyl groups are eliminated.
3.3. Textural properties of 3D macroporous srGO-based sorbents
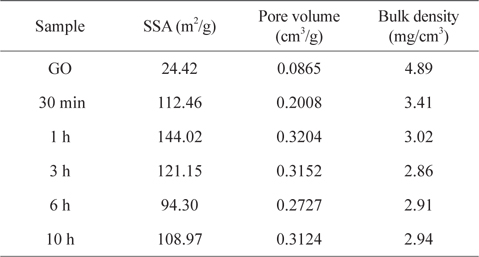
The textural properties of porous carbons such as SSA and pore volumes, which are usually measured by the N2 gas adsorption-desorption experiment and analyzed by the BET equation and the BHJ method, have been used to describe the absorption capture of oils and organic solvents. However, the contribution of SSA to the absorption capabilities in the 3D carbon-based architectures appears to be controversial. This is because the conventional SSA measurement using the N2 adsorption isotherm is merely useful for evaluating micro- or mesopores smaller than 50 nm. In this work, the SSA values of the samples as a function of reduction period, is also insufficient to plausibly explain the sorption saturation obtained from the sample thermally reduced for 1 h (it had the highest SSA of 144 m2g−1) (Table 1 and Fig. S4). The decrease of SSA from the samples reduced for 6 h is understood to be related to the partial overlapping and coalescence of srGO sheets [24]. Because the 3D macroporous srGO macrostructures are framed within sparse networks of thin, few-layered GO sheets by the ice-templating assembly, the relatively low SSA values of the samples are attributed to the high porosity contributed by macropores between the thin GO walls. Moreover, the mesopores of srGO sorbents originated from the exfoliation of few-layered GO sheets induced by the evolution of gas species from the decomposition of oxygen-functional groups and physisorbed water [5,11,18]. Bulk density can be correlated with the macroporosity and thus, the low bulk density of porous materials with the small volume of mesopores is indicative of the large macropore volume (Table 1). The variations in the bulk densities of samples are attributed to the loss of residual water and the pyrolysis of the oxygen functionalities generating the gas species (CO and CO2). As confirmed by TGA and DSC (Fig. 5a), thermally unstable GO is observed to lose up to 26% of its mass as the temperature approaches 184℃ due to the vaporization of residual water physisorbed onto its surfaces and intersheets. When the heating temperature reaches 184℃, there is a dramatic loss of mass (43%) corresponding to the decomposition of oxygen-containing groups. Such a sharp mass loss at the onset temperature can be accompanied by a strong exothermic DSC peak. Because the 3D macroporous structures are pre-framed with the thin few-layered GO sheets through the ice-templating step prior to the post-heating step, the 1-h reduction process is thought to be long enough to open most of the mesopores of the srGO by removing the residual water and the oxygen-functional groups (mainly the hydroxyl groups). It is also noteworthy that the saturated sorption values for oils and organic solvents are consistent with the steady values in the bulk densities of samples plotted versus the reduction time (Fig. 5b).
[Table 1.] Textural properties and bulk densities of samples as a function of reduction time
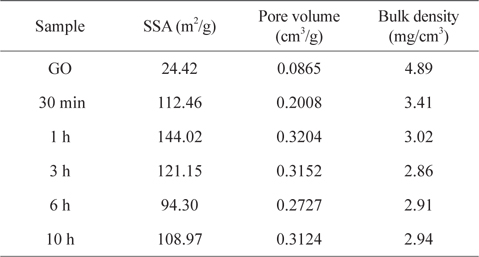
Textural properties and bulk densities of samples as a function of reduction time
3.4. Sorption parameters of 3D macroporous srGO-based sorbents
Based on the results mentioned above, the absorption parameters (e.g., reduction degree, hydrophilicity, SSA, and macroporosity) were found to play key roles in determining the absorption capabilities of the 3D macroporous srGO-based sorbents for removal of oils and organic solvents. In particular, the srGO sorbents, which were prepared through the vacuum-assisted thermal reduction for 1 h, showed saturation, where the absorption capacity was not increased by a longer reduction time. This finding indicates that the large mesoporosity of the srGO sorbents, of which the pores were opened by removing the residual water and the hydroxyl groups, as well as the hydrophobicity and C/O ratio, are crucial for achieving maximum capacity. The slight reduction that occurs under vacuum for 1 h is sufficient to create the full mesoporosity of the srGO (as shown in the largest SSA and pore volume), and moderate macroporosity comparable to that of the srGO thermally reduced for 10 h (as demonstrated by the steady value of the bulk density). In order to verify the importance of the macroporosity, we measured the absorption capacities of chemically reduced 3D rGO with a relatively higher value of SSA (289 m2g−1), C/O ratio of 8.46, and the bulk density of 18 mg/cm3(Fig. S5) [12]. As expected, the chemically reduced 3D rGO showed relatively lower absorption capacity (106× its weight) for the solvent chloroform, which was the average from five samples. Moreover, emptying the meso- and macropores in the srGO networks can facilitate the diffusion of absorbates through the 3D networks and expand the pore volumes available for physical loading of oils and organic solvents [1]. The importance of the open meso- and macropores for physical loading is supported by the fact that the absorption capacity of the srGO could not be improved by reduction beyond 1 h, even though the formation of graphitic domains on the surfaces of the hydrophobic srGO reinforces the hydrophobic interactions with the carbon chains and aromatic rings of oils and organic solvents.
In summary, we demonstrated that control over the degree of reduction of highly macroporous srGO-based sorbents by heating time under mild conditions (1 × 10−2 Torr and 200℃), results in different C/O ratios, hydrophobicities, SSAs, and bulk densities. The hydrophilic srGO sorbents thermally reduced for 1 h, achieved high pollutant-loading capacities (up to 319× their weight), where absorption capacity was not enhanced by lengthening the reduction period; rather, it remained saturated. This study on the series of absorption parameters proposed the physical mass-loading of oils and organic solvent pollutants within the pores. In addition to the hydrophobicity, the open meso- and macropores of the srGO sorbents is significant for determining the absorption capability. Therefore, the excellent capacity of the 3D srGO-based sorbents is attributed to the open pores, and correlated with the light bulk density below 3 mg/cm3 and the partial reduction of GO scaffolds (C/O ratio) slightly higher than ‘4’).